Abstract
The aim of this study was to evaluate the effects of fimasartan/amlodipine fixed-dosed combination (F/A) on left ventricle (LV) systolic function and infarct size in the rat myocardial infarction (MI) model. We induced MI in 20 rats by ligation of the left anterior descending coronary artery and they were divided into two groups [MI group (n=10) vs. MI+F/A 10 mg/kg group (n=10)]. F/A was administered for 28 days between day-7 and day-35 in the MI+F/A group and echocardiography was performed at day-7 and at day-35 after the induction of MI. Picrosirius red staining was performed to confirm the fibrotic tissue and infarct size was measured using image analysis program for Image J. At the 35-day follow-up, the LV ejection fraction (EF) was significantly higher (38.10±3.92% vs. 29.86±4.56%, p<0.001) and delta (day-35 minus day-7) EF was significantly higher (0.14±2.66% vs. −8.53±2.66%. p<0.001) in the MI+F/A group than the MI group. Systolic blood pressure was significantly lower in the MI+F/A group than the MI group (103.23±13.35 mmHg vs. 123.43±14.82 mmHg, p<0.01). The MI+F/A group had a smaller infarct size (26.84±5.31% vs. 36.79±3.10%, p<0.01) than the MI group at the 35-day follow-up. Oral administration of F/A 10 mg/kg could improve LV systolic function and reduce infarct size in a rat MI model.
Cardiac fibrosis is a typical phenomenon that occurs during cardiac muscle remodeling due to various heart diseases such as myocardial infarction (MI) and cardiac hypertrophy. Many experimental mouse models, such as permanent or temporary coronary occlusion, are commonly used in the field of cardiovascular research to increase cardiac fibrosis in the myocardium.12 In these models, myocardial remodeling occurs as MI progresses. This is accompanied by the formation of fibrotic tissue, which plays a decisive role in determining myocardial systolic and diastolic function.3 Therefore, the detection and quantification of myocardial fibrotic tissue after post-MI is an important factor in myocardial remodeling analysis.
In clinical practice, MI is undergoing various treatments such as cardiovascular surgery, stent implantation, and thrombolysis, and there is a lot of interest and research into secondary prevention through many medications.4 After reperfusion therapy for MI, β blockers, angiotensin converting enzyme inhibitors and angiotensin receptor blockers (ARBs) are used to prevent cardiovascular ischemia and to maintain cardiac function.567
Dukarb® (Boryung Pharmaceutical Co., Ltd., Seoul, Republic of Korea) is a fixed-dose combination of fimasartan (ARB) and amlodipine (calcium channel blocker). Recent clinical and experimental research has shown that ARBs have a cardioprotective effect that exceed their ability to lower blood pressure (BP), such as anti-vascular agents, and target organ protection.8 In addition, amlodipine is a long-lasting, dihydropyridine CCB that is now widely used for cardiovascular disease in lowering BP. Recently, amlodipine has been effectively used in combination with other classes of antihypertensive agents to reduce BP and cardiovascular risk.9
So far, little data is available about the effects of fimasartan/amlodipine fixed-dosed combination (F/A) on the cardioprotective effects. Therefore, the aim of this study was to evaluate the effects of F/A on left ventricular (LV) systolic function and infarct size in a rat MI model.
Sprague-Dawley male rats (Samtako, Seoul, Republic of Korea), weighing 250–260 g, aged 8 weeks, were used in all experiments. We induced MI in 20 rats and they were divided into two groups [MI group (n=10) vs. MI+F/A 10 mg/kg group (n=10)] (Fig. 1). The Ethics Committee of the Chonnam National University Medical School and the Ethics Committee of the Chonnam National University Hospital (CHU IACUC-H-2017-23) approved animal experiment protocol.
MI was induced with a slightly modified Fliss method.10 Sprague-Dawley rats were anesthetized with femoral intramuscular injection with a ketamine (50 mg/kg) and xylazine (5 mg/kg). Anesthetized rats were fixed to the operating table with tape. In addition, the respiration was continuously maintained through the ventilator (Model 683, Harvard apparatus, USA). To induce MI, the middle left anterior descending coronary artery was ligated with 5-0 silk to the myocardial region. Finally, the heart was originally positioned in the thoracic cavity, and closed the skin with 1-0 silk. A heating pad (temperature at 37±0.5℃) was used to maintain the body temperature and to increase the survival rate of the rats.
F/A (30/5 mg) was pulverized in a tablet grinder and resolved in 10 ml of water. In addition, the vortex (Vortex-Genie 2, Scientific Industries) was used for uniformity of drug before administration of F/A. The body weight was measured daily before the administration of the drug and was administered proportionally to the body weight (10 mg/kg). The F/A was administered by oral injection using zonde needles (1.2×80 mm, 18 G) for 28 days between day-7 and day-35 after the induction of MI (Fig. 1).
A conventional echocardiography was performed according to the main laws of the American Society of Echocardiography.11 Echocardiography was checked in supine position after shaving the chest hair of the subjects. We acquired short-axis images of the LV by transthoracic echocardiography, M-mode and two-dimensional echocardiography images were obtained from the papillary muscle level using echocardiography machine (15-MHz linear array transducer, iE33 system, Philips medical systems).12 Echocardiography was performed at baseline, at day-7, and at day-35 (Fig. 1).
After opening the thoracic cavity, cardiac arrest was induced by administering potassium perchlorate (KClO4) to the thoracic aorta. After heart tissue extraction, saline was used to remove blood from the LV. Cardiac tissue was fixed in 4% paraformaldehyde solution for 3 days, and fixed in paraffin. The tissue fixed to the paraffin was cut to a thickness of 4 µm. Picrosirius red staining was performed to confirm the fibrotic tissue and scar formation in the heart. The infarct size was measured using image analysis program for Image J.13
Systolic BP was measured with the tail-cuff method (Visitech Systems, BP 2000).14 Rats were trained to be able to measure BP more than three times per week before drug administration. Systolic BP was measured when the rats were awake before they were scarified at day-35.
We used Statistical Package for the Social Sciences (SPSS) 22.0 for Microsoft Windows (SPSS, Inc., Chicago, IL, USA) for all statistical analyses. All numerical variables were presented as mean value±standard deviation (SD) and were compared by independent samples t-test. A p value <0.05 was considered statistically significant.
The mortality rate after MI induction was 40% overall, and the mortality rate during the follow-up period was about 30%.
LVEF was measured in a total of twenty rats. A LV functional evaluation was assessed at day-7 and day-35 follow-ups after the induction of MI (Table 1). LV internal diameter at diastole and LV internal diameter at systole were similar between the MI group and the MI+F/A 10 mg/kg group (Fig. 2A, B). Fractional shortening was significantly greater (16.18±1.99% vs. 12.30±2.17%, p<0.001) and LVEF (38.10±3.92% vs. 29.86±4.56%, p<0.001) was significantly higher in the MI+F/A 10 mg/kg group than the MI group (Fig. 2C, D). The delta EF was obtained by subtracting the day-7 measurement from the day-35 measurement. Delta EF was significantly higher in the MI+F/A 10 mg/kg group than the MI group (0.14±2.66% vs. −8.53±2.49%, p<0.001) (Fig. 2E).
Systolic BP was not significantly different between the MI group and the MI+F/A 10 mg/kg group at day-7 after the induction of MI (110.11±14.82 mmHg vs. 111.00±13.35 mmHg, p=0.87). There was no significant difference in pulse rate between the MI group and the MI+F/A 10 mg/kg group (381.07±50.54 BPM vs. 386.17±43.21 BPM). However, at the day-35 follow-up, systolic BP was significantly decreased in the MI+F/A 10 mg/kg group compared to the MI group (103.23+13.35 mmHg vs. 123.43+14.82 mmHg, p<0.01).
High color contrast is an important factor in the measurement of fibrotic tissue. Scar formation of cardiac muscle after MI was identified as pricosirius red staining (Fig. 3A) which is marked as red in the fibrotic tissue within the scar in the heart muscle, and is marked as yellow in the non-infarcted tissue. The infarct size was quantified using the Image J program. The MI+F/A 10 mg/kg group had a significantly smaller infarct size than the MI group (26.84±5.31% vs. 36.79±3.10%, p<0.01) (Fig. 3B).
The present study was conducted to evaluate the effects of F/A on LV systolic function and cardiac fibrosis in a rat MI model. F/A fixed-dose combination treatment after the induction of MI significantly improved LV systolic function and significantly reduced infart size.
After MI development, the heart undergoes a series of pathologic remodeling processes, resulting in heart failure and cardiac death.15 After MI, myocardial reconstruction can have a significant impact on the function of LV and prognosis for survival. The expansion of the infarct is followed by a long-term growth phase of fibroblast proliferation and collagen deposition. Also, the longer the period, the more collagen deposition is accumulated and the infarct area becomes thin and elongate.16
The ventricular enlargement process for early MI can be influenced by three interdependent factors : infarct size, infarction healing, and ventricular wall stress.17 Angiotensin II is signaled through the angioensin II type 1 receptor (AT1) and angiotensin II type 2 receptor (AT2).18 Recent studies have indicated that AT2 receptor stimulation interferes with the deleterious effects of AT1 signaling in fibrotic diseases.19 Activation of this compensation system reduces inflammation and collagen deposition.20
Fimasartan (BR-A-657-K, KANARB®) is a new ARB developed for the first time in Korea. It is a selective AT1 receptor blocker and has shown rapid and potent antihypertensive effects in clinical trials.21222324 Han et al.8 reported that fimasartan preconditioning has the potential to suppress myocardial ischemia/reperfusion injury and these beneficial effects could prevent the mitochondrial dysfunction and apoptosis accompanied by ischemia/reperfusion injury. Lim et al.25 reported that fimasartan attenuates cardiac remodeling and dysfunction in rats after MI and may prevent the progression to heart failure after MI and myocardial fibrosis and inflammation were downregulated by fimasartan.
Amlodipine is a dihydropyridine CCB that is widely used for the treatment of hypertension. Clinical and experimental studies have shown that amlodipine has some BP-independent effects, including reducing risk of adverse cardiovascular events in patients with coronary heart disease without hypertension, attenuating endothelial dysfunction, and alleviating ischemia-reperfusion-induced heart injury.262728
The present study demonstated that F/A fixed-dose combination therapy improved LV systolic function and reduced infarct size effectively after the induction of MI. The cardioprotective effects of F/A seems to result from the additive beneficial effects of fimasartan and amlodipine for cardiac fibrosis and collagen deposition, vascular inflammation, and endothelial function. The present study suggests that F/A treatment can be used as an effective treatment option for preventing LV remodeling and improving LV function in patients with MI.
There are several limitations to be mentioned. First, the study group had to be divided into four groups including a fimasartan only group and amlodipine only group for the evaluation of the synergistic effects of fimasartan and amlodipine. Second, we only focused on the morphological changes of the heart. Furthermore, the molecular part of the morphological change was not evaluated. Third, we observed the effects of F/A on LV systolic function and cardiac fibrosis for only 1-month after the induction of MI. Therefore, further long-term study is needed.
In conclusion, oral administration of F/A 10 mg/kg could improve LV systolic function, lowered blood pressure and reduced infarcted tissue area in rat MI model suggesting its clinical utility as an effective treatment option in patients with MI. Further investigations regarding this drug have to be conducted and its efficacy remains to be proven in larger animal models.
Figures and Tables
FIG. 2
Changes in cardiac parameters by echocardiography. (A) Left ventricle internal diameter at diastole (LVIDd), (B) left ventricle internal diameter at systole (LVIDs), (C) fractional shortening (FS), (D) ejection fraction (EF), (E) delta EF. MI: myocardial infarction, F/A: fimasartan/amlodipine. ***p<0.001. FIG.
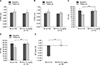
FIG. 3
(A) Picrosirius red stain and (B) measurement of infract size (%). MI: myocardial infarction, F/A: fimasartan/amlodipine. **p<0.01.
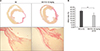
TABLE 1
Echocardiographic findings

Data is mean±SD. IVSd: interventricular septal thickness at diastole, IVSs: interventricular septal thickness at systole, LVIDd: left ventricle internal diameter at diastole, LVIDs: left ventricle internal diameter at systole, LVPWd: left ventricle posterior wall thickness at diastole, LVPWs: left ventricle posterior wall thickness at systole, EDV: end-diastolic volume, ESV: end-systolic volume, EF: ejection fraction, SV: stroke volume, FS: fractional shortening.
ACKNOWLEDGEMENTS
This study was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) & funded by the Korean government (MSIT) (2018M3A9E2024584) and a grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI13C1527).
References
1. Katz MG, Fargnoli AS, Gubara SM, Chepurko E, Bridges CR, Hajjar RJ. Surgical and physiological challenges in the development of left and right heart failure in rat models. Heart Fail Rev. 2019; 24:759–777.

2. Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, et al. American Heart Association Council on Basic Cardiovascular Sciences. Council on Clinical Cardiology. Council on Functional Genomics and Translational Biology. Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res. 2012; 111:131–150.
3. Emde B, Heinen A, Gödecke A, Bottermann K. Wheat germ agglutinin staining as a suitable method for detection and quantification of fibrosis in cardiac tissue after myocardial infarction. Eur J Histochem. 2014; 58:2448.

4. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. Authors/Task Force Members. Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016; 18:891–975.

5. Soumerai SB, McLaughlin TJ, Spiegelman D, Hertzmark E, Thibault G, Goldman L. Adverse outcomes of underuse of beta-blockers in elderly survivors of acute myocardial infarction. JAMA. 1997; 277:115–121.

6. Køber L, Torp-Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, et al. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med. 1995; 333:1670–1676.

7. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003; 348:1309–1321.

8. Han J, Park SJ, Thu VT, Lee SR, Long le T, Kim HK, et al. Effects of the novel angiotensin II receptor type I antagonist, fimasartan on myocardial ischemia/reperfusion injury. Int J Cardiol. 2013; 168:2851–2859.

9. Owen AJ, Reid CM. Cardio classics revisited: focus on the role of amlodipine. Integr Blood Press Control. 2012; 5:1–7.

10. Fliss H, Gattinger D. Apoptosis in ischemic and reperfused rat myocardium. Circ Res. 1996; 79:949–956.

11. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978; 58:1072–1083.

12. Gardin JM, Siri FM, Kitsis RN, Edwards JG, Leinwand LA. Echocardiographic assessment of left ventricular mass and systolic function in mice. Circ Res. 1995; 76:907–914.

13. Zhang Y, Köhler K, Xu J, Lu D, Braun T, Schlitt A, et al. Inhibition of p53 after acute myocardial infarction: reduction of apoptosis is counteracted by disturbed scar formation and cardiac rupture. J Mol Cell Cardiol. 2011; 50:471–478.

14. Buñag RD. Validation in awake rats of a tail-cuff method for measuring systolic pressure. J Appl Physiol. 1973; 34:279–282.

15. Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000; 101:2981–2988.

16. Fishbein MC, Maclean D, Maroko PR. The histopathologic evolution of myocardial infarction. Chest. 1978; 73:843–849.

17. Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990; 81:1161–1172.

18. de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000; 52:415–472.
19. Rompe F, Artuc M, Hallberg A, Alterman M, Ströder K, Thöne-Reineke C, et al. Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor kappaB. Hypertension. 2010; 55:924–931.

20. Steckelings UM, Paulis L, Namsolleck P, Unger T. AT2 receptor agonists: hypertension and beyond. Curr Opin Nephrol Hypertens. 2012; 21:142–146.
21. Lee SE, Kim YJ, Lee HY, Yang HM, Park CG, Kim JJ, et al. Investigators. Efficacy and tolerability of fimasartan, a new angiotensin receptor blocker, compared with losartan (50/100 mg): a 12-week, phase III, multicenter, prospective, randomized, double-blind, parallel-group, dose escalation clinical trial with an optional 12-week extension phase in adult Korean patients with mild-to-moderate hypertension. Clin Ther. 2012; 34:552–568. 568.e1–568.e9.

22. Lee H, Yang HM, Lee HY, Kim JJ, Choi DJ, Seung KB, et al. Efficacy and tolerability of once-daily oral fimasartan 20 to 240 mg/d in Korean Patients with hypertension: findings from Two Phase II, randomized, double-blind, placebo-controlled studies. Clin Ther. 2012; 34:1273–1289.

23. Park JB, Sung KC, Kang SM, Cho EJ. Safety and efficacy of fimasartan in patients with arterial hypertension (Safe-KanArb study): an open-label observational study. Am J Cardiovasc Drugs. 2013; 13:47–56.

24. Lee H, Kim KS, Chae SC, Jeong MH, Kim DS, Oh BH. Ambulatory blood pressure response to once-daily fimasartan: an 8-week, multicenter, randomized, double-blind, active-comparator, parallel-group study in Korean patients with mild to moderate essential hypertension. Clin Ther. 2013; 35:1337–1349.

25. Lim BK, Park JJ, Park SJ, Lee YJ, Kwon JS, Kim EJ, et al. Fimasartan for remodeling after myocardial infarction. J Clin Med. 2019; 8:E366.

26. Nissen SE, Tuzcu EM, Libby P, Thompson PD, Ghali M, Garza D, et al. CAMELOT Investigators. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA. 2004; 292:2217–2225.

27. Mason RP, Kubant R, Heeba G, Jacob RF, Day CA, Medlin YS, et al. Synergistic effect of amlodipine and atorvastatin in reversing LDL-induced endothelial dysfunction. Pharm Res. 2008; 25:1798–1806.

28. Khan NA, Chattopadhyay P, Abid M, Pawdey A, Kishore K, Wahi AK. Protective effects of amlodipine on mitochondrial injury in ischemic reperfused rat heart. J Environ Biol. 2012; 33:591–595.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download