Abstract
Today, the incidence of cancer in the world is rising, and it is expected that in the next several decades, the number of people suffering from cancer or (the cancer rate) will double. Cancer is defined as the excessive and uncontrolled growth of cells; of course (in simple terms), cancer is considered to be a set of other diseases that ultimately causes normal cells to be transformed into neoplastic cells. One of the most important causes of the onset and exacerbation of cancer is excessive oxidative stress. One of the most important proteins in the inner membrane of mitochondria is Reactive Oxygen Species (ROS) Modulator 1 (ROMO1) that interferes with the production of ROS, and with increasing the rate of this protein, oxidative stress will increase, which ultimately leads to some diseases, especially cancer. In this overview, we use some global databases to provide information about ROMO1 cellular signaling pathways, their related proteins and molecules, and some of the diseases associated with the mitochondrial protein, especially cancer.
Cancer consists of a series of diseases of which the main feature is the unregulated cell growth, invasion, and diffusion of cells from their original location and places to the rest of the body. A malignant tumor is composed of cells that are significantly different from normal cells, morphologically, and physiologically.1 Neoplasm is the leading cause of death in industrialized countries and is the second leading cause of mortality in developing countries.2 The main reason for an increase in cancer in developed countries is the aging of demographic tissue, the spread of dangerous behaviors such as smoking, lack of exercise, and western food habits.3 In 2008, there were about 13 million cases of cancer and about 8 million deaths worldwide.3 Key genes that fail in cancer are divided into five groups: 1) Proto-carcinogens (proto-oncogenes) 2) DNA repairing genes 3) Tumor suppressor genes 4) Genes involved in apoptosis and 5) Genes involved in telomere homeostasis.456 Any disruption in the function of any of these genes can lead to cancer.456 In addition to these cases, currently, another important factor that researchers consider as an important part of starting and exacerbating cancer is increasing oxidative stress and many studies have been carried out on this around the world. Researchers have used many markers for diagnostic, therapeutic, and prognostic evaluation of cancers,78 however, because of diversity in the molecular mechanisms, new markers are always needed.
In this overview, we use international databases such as Pubmed, Google scholar, Scopus and Web of sciences. By using keywords ROMO1, oxidative stress, ROS, cancer, and diseases, we could find information about the ROMO1 protein, its associated signaling pathways, and the diseases in which this protein might interfere with them, especially cancer.
Reactive Oxygen species (ROS) are endogenously generated by the electron transfer route in the mitochondria, as well as in other metabolic directions.910 External and environmental factors are also involved in the development of ROS, such as chemicals and types of radiation.111213 ROS plays different key roles in the normal cell process, so a particular amount of ROS is required for normal cell operation (function).12 The accumulation and change in the level of these molecules can have a key role in the cellular activity, which is related to the concentration of ROS in the cell. ROS in the cell can cause excessive activation of some signaling pathways, where they convert normal cells into cancer cells.12 ROS in cancer cells can cause tumor growth, cellular invasion, metastasis, and angiogenesis and eventually leads to neoplasm or other diseases.1113 Generally, altered levels of ROS can cause cell damage, inflammation, and mutation to DNA, all of which mau ultimately end up with cancer.12 These molecules are responsible for more than 95% of the tissue damages at the molecular level, which can lead to cancer.14
Reactive Oxygen Species Modulator 1 (ROMO1) is one of the proteins involved in the production of ROS by complex III of the mitochondrial electron transport chain. Other names for this protein are hGlyrichin, Epididymis tissue protein Li 175 and Mitochondrial targeting GxxxG motif protein (MTGM2), and the mitochondrial protein are made up of 79 amino acids and its chromosomal location is 20q11.22. In 2018, Lee et al.15 proposed different roles for this protein. They determined that Homo sapiens Romo1 has two transmembrane domains (TMDs), each of which consists of an α helix and are joined by a basic loop. They stated that TMD1 has a hydrophobic α helix, but TMD2 consists of polar amino acids (K58, T59, Q62, S63, T66, and T69). These amino acids are individually separated by other amino acids, and there are 3.6 amino acids in α-helical turn. It was suggested that the amino acid sequences of ROMO1 in 82 of 247 animal species were almost identical with that of H. Sapiens Romo1, which indicates that the sequence is protected among them. Due to the small size and the hydrophobic nature of ROMO1, they were not able to identify the structure of this important mitochondrial protein by using x-ray crystallography or nuclear magnetic resonance (NMR) imaging methods, but by using structural bioinformatics, they proposed ROMO1 structure, as a hexameric model.15
Lee et al.15 showed that Romo1 could be formed in the membranes of homo-oligomers in the inner membrane of mitochondria. They are considered a family of virus-encoded non-selective ion channels, class II viroporin, that includes small α-helical transmembrane proteins with one amphipathic helical TMD in order to determine the role of biophysical ROMO1 in the mitochondrial membrane. In these types of channels, there are two TMDs, one amphipathic helical TMD, which is required for these channels to build homo-oligomeric non-selective ion channels. Due to this similarity in the structure of ROMO1 and class II viroporin, they suggested that ROMO1 could be a viroporin-like eukaryotic ion channel. Since it has been shown that ROMO1 can be as a viroporin-like eukaryotic ion channel, therefore, the homo-oligomeric form of ROMO1 can cause permeability in mitochondria membranes, like viroporins. In the structure of ROMO1, mentioned above, which consists TMDs, C-terminal amphipathic helix TMD2 in this structure is required for the pore-forming activity. As mentioned, ROMO1 could have the same role like viroporins, and the viroporin-like eukaryotic ion channel; therefore, ROMO1 can be inhibited by viroporin inhibitors, such as hexamethyleneamiloride (HMA) but not by amantadine, rimantadine, or N-nonyldeoxynojirimycin. On the other hand, by using viroporin inhibitors, HMA, the membrane potential (ΔΨm) increases by the inhibition of ROMO1. However, it has been shown that this mitochondrial protein can act as a non-selective cation channel like class II viroporins. We know that some transition metal ions (Cu2+, Zn2+, and Fe2+) are very important in mitochondrial physiological activity, such as controlling the production of ROS. Lee et al.15 found that in the mitochondria membrane, Fe2+ can be used to control and reduce the activity of ROMO1. Generally, they concluded that ROMO1 could act as a hexameric, non-selective cation channel in a manner analogous to viroporins. Na et al.16 used the Romo1 siRNA, inhibit extracellular signal-regulated kinases (ERK) pathway that plays a role in cell proliferation, which was activated by Romo1-induced ROS. The role of this important mitochondrial protein in this study reconfirmed.16 ROMO1 can stimulate cellular proliferation by producing ROS and activating some of the various signaling mediators, such as ERK,16 TGF-β route, and their factors, which consist of Smad2/3, extracellular matrix (ECM) proteins, and Epithelial mesenchymal transition (EMT) factors;17 therefore, ROMO1 can affect these signaling pathways. Dickkopf1 (DKK1) is an important protein that can inhibit and interact with signaling pathways such as Wnt/B-catenin, as well as DKK1 affects ROMO1 gene expression and inhibits its gene.18 Spermine is one of the most important polyamines that is involved in cellular protection and the protection of membrane function and structure. ROMO1 can also be associated with spermine because during the research on the antioxidant effect of spermine. They found that spermine can not only have anti-apoptotic effects but it could also lead to fewer numbers of ROS by reducing the expression of ROMO1 gene; therefore, this research shows that ROMO1 can associate with polyamins such as spermine and affect fertility.19 Matrix metalloproteinases (MMPs) acts in an especially key role in the regeneration and improvement of an organ in living organisms.20 It has been determined that MMPs activity can be set to ROS, which itself increases through some pathways and factors such as growth factors, cytokines, tumor promoters and ROMO1, so ROMO1 can effect MMP and activate these enzymes through the production of ROS.21 ROMO1 affects nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) route, the mitochondrial protein that can increase ROS and this increase of ROMO1-induced ROS can be achieved by controlling and decreasing the inhibition of κB kinase (IKK); therefore, the NF-κB pathway can be activated, which will cause invasion and proliferation in cancer cells (Fig. 1).22 Moreover, in 2015, Shyamsunder et al.,22 conducted research and proved that ROMO1, in addition to the direct impact on the NF-κB pathway by ROMO1-induced ROS, could result in the production of epithelial to mesenchymal transition (EMT) factors from the NF-κB pathway and in this study EMT factors such as SNAIL1, 2 and MMP2 were increased as well. As we know, the EMT process can cause invasion and metastasis through damaging the cell-to-cell connections and losing the cell adhesion, therefore, ROMO1 can result in an invasion and metastasis by inducing NF-κB-driven EMT factors (Fig. 1).22 One of the (most) important regulatory genes and protooncogenes in cell cycle activity and mitosis is the Myc family. The Myc family includes three associated human genes:, l-myc, n-myc and c-myc. This protein is increased in response to cell division and mitosis, and its expression level is strongly controlled by transcriptional, translational and post-translational mechanisms. The main mechanism for the loss of this protein is ubiquitin-mediated proteolysis. Myc is poly ubiquitylated via E3 ubiquitin ligases that consists of Skp2, the F-box proteins and Fbw7. It is known that Skp2 can restrain some Cdk inhibitors and tumor suppressor proteins such as p27Kip1, p57Kip2, p130 and Tob1. In addition, previous studies shown that Skp2 is overexpressed in cancer cells. As the role of the two proteins (Skp2 and Myc) mentioned, Lee et al.23 showed a link between ROMO1, Skp2 and Myc. They introduced a new pathway as a negative feedback during G1 phase for Myc. They showed that if a cell is stimulated for mitosis, the level of Myc protein will increase for cell division. With increasing (the amount of) Myc, the expression of ROMO1can be enhance, as we know; an increase of ROMO1 can result in excessive release of ROS. ROMO1-induced ROS, eventually it can cause the cytoplasmic translocation of Skp2 for the degradation of Myc by ubiquitylation. For this reason, ROMO1 is introduced as a negative feedback regulator for Myc ubiquitylation.23 As mentioned, this mitochondrial protein presents in the mitochondrial inner membrane that can affect dynamic and mitochondrial shapes. The overlapping with The M-AAA Protease 1 Homolog (OMA1) gene encodes a zinc-dependent metalloendopeptidase, which is an enzyme that is dependent on the mitochondrial inner membrane and contains 524 amino acids.2425 The mitochondrial inner membrane has two proteases called m-AAA (AFG3L2) and i-AAA (YME1L), the type m-AAA is located in the inner membrane of the mitochondria and type i-AAA is in the inter membrane space.2425 m-AAA protease 1, in function, overlaps with enzyme OMA1.2425 On the other hand, there are some GTPases in the mitochondrial membrane, such as mitofusins and Optic Atrophy Protein 1 (OPA1), which are involved in regulating and dynamically balancing mitochondrial membranes.2425 OPA1 protein has different isoforms that are generated by two proteins, OMA1 and YME1L,2425 and are located in the mitochondrial membrane, which contains various isoforms that cause the dynamic regulation of mitochondria.2425 To be more precise, m-AAA makes pre-pro-OMA1 to the pro-OMA1, and pro-OMA1 along with YME1L affect different locations of OPA1 and create OPA1 isoforms.2425 Recently, in one study on cell culture, researchers defined the role of ROMO1 as a protein that has translocase properties and with losing the activity of ROMO1, the processing of OPA1 and mitochondrial dynamics are affected.26
Now, if event mutations in the genes of these proteins are involved in the development of OMA1, conditions of oxidative stress will be provided, which eventually results in the increased activity of the enzyme OMA1 and excessive production of one of the isoforms of OPA1, especially short-isoforms, and will lead to mitochondrial degradation.2425 There are several essential proteins in mitochondrial membranes. The important proteins in the internal membrane are known as the Tim17 family of proteins that include Tim17, Tim22, and Tim23 and in the outer membrane are known as TOM22, TOM20 and TOM40. These mitochondrial proteins play a key role in the translocation of materials from the width of mitochondrial membranes. In 2016, it was proved by Žárský and Doležal27 that the human ortholog ROMO1 has special homology with Tim17 family, so ROMO1 has some Tim17-like functions in the inner membrane of mitochondria. One of the most important molecules and mediators in inflammation is Tumor necrosis factor-a (TNF-a). On TNF-a signaling route, there are two main pathways. One of these pathways ends with inflammation and survival, which in this route, complex 1 can launch mitogen-activated protein kinase (MAPK) and NF-κB. Another way that ends in apoptosis is through the activation of complex II where in ROS, a caspase cascade and the mitochondria operation are called downstream molecules. It has been shown in the research that ROMO1 can be a molecular mediator between TNF-α and activation of the pathway of apoptosis through an effect on TNF-a signaling pathway. Components of complex II, which include Fas-associated death domain protein (FADD), TNF receptor-associated protein with death domain (TRADD), pro-caspase-8, TNF receptor-associated factor 2 (TRAF2), receptor interacting protein 1 (RIP1), and TNF receptor-associated protein with death domain (TRADD), can bind to C-terminal of ROMO1 molecule. In the next section, ROMO1 by using B-cell lymphoma-extra large (Bcl-XL), can decrease the mitochondrial membrane potential, which produces ROS, and ROMO1-induced ROS can increase the activation of c-Jun N-terminal kinase (JNK) and eventually inducing apoptotic cell death. Therefore, ROMO1 is involved in other important molecular signaling (apoptosis) and in association with TNF-a, RIP1, TRADD, TRAF2, FADD, pro-caspase-8, Bcl-XL, ROS, JNK molecules (Fig. 1).28
ROS at low levels play a key role in the growth and proliferation of normal cells; therefore, their essential role in the cell cycle, from G1 to S, should not be overlooked. One of the most important cyclin dependent kinase inhibitors (CKIs) is p27KIP1.29 p27KIP1 is another tie molecule in antioxidant-associated cell cycle stop. As stated above, ROMO1-induced ROS can be essential for normal cell proliferation from G1 to S in the cell cycle, but there is a regulator in the transmission and completion of the cell cycle and this regulator molecule is p27Kip1. For this reason, it can be said that not only does ROMO1 contribute to the production of optimum levels of ROS in proliferation of normal cells, but it also can associate with some regulatory molecules in the pathway of a cell cycle like p27Kip1.30 As mentioned earlier, ROMO1 can be associated with planned cell death characterized by DNA chopping and apoptotic bodies (apoptotic pathways). More precisely, in different cell lines, it has been shown that increased expression of ROMO1 could enhance ROMO1-derived ROS. As a result, this led to an increase in the releasing of cytochrome C of the mitochondria and triggering the caspases which act a key function in apoptosis through triggering kinases like c-Jun N-terminal kinase (JNK), apoptosis regulating kinase 1 (ASK1) and p38, which eventually ends in cellular apoptosis and cell death.31 Another interfering mecxhanism of ROMO1 in cell cycle is the activation of G2/M and diminishing phospho-cdc2, that with its relationship between G2/M and phospho-cdc2 can promote proliferation in cancer cells.32 Intra cellular conditions, temperature, and pH can affect the level of gene expression and ROMO1 protein, as it has been observed that the level of Romo1 is enhanced in acidic pHs.33 Since ROMO1 can make a balanced state of oxidative stress, its absence, such as with its over expression, can be a problem in the mitochondrial membrane. In the absence ROMO1, OPA1 can't be well oligomerized, and this will damage the mitochondrial membrane and eventually lead to the enhancement of oxidative stress.34 A scavenger receptor (SRA) is one of the important subunits of scavenger receptors, which increases inflammatory responses and infections. It has been shown that by stimulating this protein, the ERK pathway will be activated. SRA could increase neutrophil extracellular traps (NETs). NETs are factors and are released by neutrophils to fight infectious agents; therefore, these agents have antimicrobial properties. SRA uses the ERK pathway to activate NADPH oxidase 2 (NOX2) and ROMO1 and the activation of these two proteins can increase ROS, which will eventually lead to NETs formation (Fig. 2). So, one of the other ways in which ROMO1 is involved is the path of activation NETs generation.35
ROMO1 is a mitochondrial protein that was first reported in cancer tissue in 2006, in which tissues, ROMO1 produced a type of drug resistance.36 ROMO1, as a ROS regulating protein, has been seen in various neoplasm cells, as well as in the invasion and has also been involved in progression of cancer cells.36 Previous studies that focused on the recurrence of some cancers after chemotherapy showed by using PCR, a gene expression of the protein has been increased in these cancerous tissues, which has been able to break through balance and can cause cancer recurrence and resistance to chemotherapy.37 In 2006, Chung et al.37 investigated different cancer cell lines associated with this protein, which increased the expression of the ROMO1 gene in these cell lines. Also, in the study, they found that the mitochondrial protein increased ROS in cells. In 2007, Hwang et al.38 researched a variety of cancer cell lines related to ROMO1, which in these cell lines that increased ROMO1-induced ROS, led to a drug resistance against 5-FU. ROMO1, in addition to other pathways, can affect MMPs through ROMO1-induced ROS, and it is possible that this important mitochondrial protein is involved in metastasis and cellular invasion of other organs.21 Chung et al.21 investigated liver cancer tissue samples and also liver cancer cell lines, which increased ROMO1 gene in cancerous tissues compared with healthy adjuvant tissues, and in hepatocellular carcinoma cell lines compared with normal cell lines there was found a similiar increase in gene expression. In 2014, Lee et al.,39 conducted research on people with lung cancer. They compared protein levels of ROMO1 in cancerous tissues with healthy individuals, and found that ROMO1 in cancer samples was significantly increased. In addition, they measured the amount of mitochondrial protein by using ELISA method in the serum of non-small cell lung cancer (NSCLC) individuals and normal subjects, and since its serum level in patients showed a significant increase compared with healthy subjects, they introduced ROMO1 as a biomarker in diagnosis NSCLC.39 Due to these proteins, ROMO1, OMA1, OPA1, m-AAA and i-AAA are involved in mitochondrial membranes, all of which are responsible for organizing and sustaining mitochondria. ROMO1 affects OPA1 and in this way affects the maintenance of the mitochondrial membrane structure. In a study by Norton et al.,34 they found that the reduction or destruction of ROMO1 could disrupt the oligomerization of OPA1, and regarding to role of OPA1 in maintaining and integrity of mitochondrial membranes, the mitochondrial membrane would be damaged. On the other hand, an increase of ROS in the cell, can activate the pathway of apoptosis by releasing cytochrome C, which ultimately results in cell death, and the enhancement of ROMO1-induced ROS trigger cancer eventually.34
We know that one of the important tumor markers used today in diagnostic laboratories is carcinoembryonic antigen (CEA). This tumor marker is significantly increased in some cancers, which is why it is used to detect various cancers, but unfortunately, the enhancement of this marker tumor cannot be attributed to just one type of cancer, so its specification is not high. Generally, today, in the world, CEA has been identified as one of the most important tumor markers for the diagnosis of malignancies and even other illnesses.40 In 2017, Chen et al.,40 conducted research in order to differentiate malignant from benign pleural effusion and concluded that in patients with malignant pleural effusion compared to benign pleural effusion, serum levels of CEA and ROMO1 have increased significantly. For this reason, they stated that this mitochondrial protein (ROMO1) could be important in distinguishing between malignant pleural effusion and benign pleural effusion as much as CEA, and ROMO1 was introduced as one of the most important proteins in the diagnosis of certain diseases, particularly in malignancy.40 As mentioned, the mitochondrial protein can have anti-chemotherapy effects, as this property was proven during a research conducted by Lee et al.41 on people with Non-small Cell Lung Cancer. They saw this anti-chemotherapeutic property because of the high expressions ROMO1, as this enhancement of mitochondrial protein led to an increase in the ROS production and could counteract the antioxidant properties of platinum.41
It has been shown that genetic polymorphisms of ROMO1 gene and their estimated haplotypes can be associated with an increased risk of gastric cancer (GC) and can make people susceptible to this deadly cancer. Of course, along with this factor for developing GC, a number of other factors, such as tobacco and alcohol consumption as well as H. Pylori infections, are mentioned.42
One of the most important and deadly cancers associated with the digestive system is colorectal cancer (CRC), which is rising in the world. In the context of its early diagnosis and good prognosis for this cancer, extensive research has been done. One recent study of this lethal cancer was carried out in 2017 by Kim et al.,36 and they showed that ROMO1 could lead to lymphatic incursion of primitive tumors in CRC, that is an especially key cause of lymphatic metastasis which suggests that increased expression and ROMO1 protein have poor prognoses for CRC.
Human glioblastoma (GBM) is another important and deadly tumor in humans, which is the most invasive and current primitive brain neoplasm and can happen at any stage of life. The average survival for GBM is about 1.2 years from diagnosis and a half-year for regression GBM despite tumor removal surgery, radiotherapy, or chemotherapy. In a study conducted on people with this malignancy, they discovered that ROMO1, by interfering in cell cycle, stimulating G2/M and reducing phospho-cdc2, promotes tumor growth and proliferation in the early stages of cancer cells in the brain. Ultimately, they introduce this protein as a new goal in treatment and detect this malignancy.32 In 2013, Kim et al.,18 investigated two lung cancer cell lines and concluded that DKK1 could be associated with ROMO1 and negatively regulated the expression of its genes, which reduced the production of ROMO1-induced ROS in cancer cell lines. Recently, researches have shown that oxidative stress can contribute to the development and pathogenesis of lung disease as well as chronic obstructive pulmonary disease (COPD) and pulmonary fibrosis, which can eventually become lung cancer. Shin et al.43 performed a study on lung tissues in individuals with idiopathic pulmonary fibrosis (IPF) and they observed that ROMO1 increased the expression of its genes and proteins in patients with IPF, and proposed that ROMO1 could induce apoptosis by the enhancement of oxidative stress in lung epithelial cells, leading to disease progression and possibly lung cancer.
Oxidative stress, in addition to starting and exacerbating growth of cancer cells, can damage sperm maturation and cause infertility. As we know the relationship between ROMO1, spermine and fertility, the management of ROMO1 can also be effective in treating some infertility problems.19 Recently, in a study on the characteristics of dog sperm, it has been shown that factors such Iodixanol supplementation can reduce the amount of ROMO1 gene expression, which ultimately leads to the reduced amount of ROS in the cell and will improve the characteristics of sperm.44
As proven in the previous studies, ROMO1 increases in diabetic conditions and can lead to tissue damage such as diabetic nephropathy (DN), which this condition can ultimately cause kidney diseases such as, fluctuations in appearance and action of kidney, including nephrotic syndrome, glomerular hypertrophy, pressure increase, excretion of albumin from urine and renal fibrosis. It has been observed that ROMO1 increases in diabetic conditions and this enhancement of the mitochondrial protein can increase ROS. John et al.45 conducted a study on diabetic nephropathy in mice in 2017. They were able to reduce the expression of genes and ROMO1 by using GYY4137 as an H2S donor, which is why this molecule is known as a potent anti-oxidant, anti-inflammatory and the protection of cell, which reduced the symptoms of diabetic nephropathy in mice; Therefore, this important membrane protein can also be involved in diabetes and its related diseases.1045 ROMO1 and ROMO1-derived ROS are two interdependent components and together, interact with different signaling pathways in various diseases.
One of the diseases in which ROMO1 and ROMO1-derived ROS were investigated, is renal interstitial fibrosis (RIF), the earlier pathological mechanism of a variety of renal diseases and causes end stage renal diseases (ESRD). It has been shown that ROMO1 can affect TGFβ signaling pathway and related factors, consisting of Smad2/3, extracellular matrix (ECM) proteins and epithelial-mesenchymal transition (EMT) factors that result in the deterioration of RIF. Liu et al.,17 suggested that this important mitochondrial protein can function as a new marker for the diagnosis and treatment for RIF.
As it has been said, cancer and type 2 diabetes mellitus (T2DM) are two of the most concerning diseases in the world, and their association with increased oxidative stress has been identified, as well as the relationship between ROMO1 and oxidative stress. One of the most important and common disorders of T2DM is diabetic retinopathy (DR). The rs6060566 polymorphism of the ROMO1 gene has been studied, so it was found that the rs6060566 polymorphism of the ROMO1 gene could be a risk factor for DR in T2DM patients.46 Nowadays, with the advent of botanical science, plants are used to treat many different diseases, and we know that important diseases are caused by disrupting the antioxidant and oxidative systems. It has been shown that oxidative stress can be reduced by using botanical science with a specific plant called mulberry leaves. Further investigations revealed that this plant reduces the amount of oxidative stress by decreasing the expression of ROMO1 gene and protein,47 so with more research, different plants that affect this mitochondrial protein which can reduce oxidative stress can be discovered and even potentially play a role in the treatement of diseases like cancer. Caveolae (“tiny caves”; accumulation of caveola), that are a kind of lipid raft, are a tiny (500–1000 Angstrom) unsheathed the cell membrane in different cells kinds, particularly embryonic cells, adipocytes, and endothelial notochord cells. These flask-liked structures have proteins, lipids such as sphingolipids, and cholesterol and perform a role in signal transferring. Caveolae plays a key role in mechano-protection, mechano-sensation, oncogenesis, and the entrance of microorganisms such as some bacteria and viruses.484950 Since oxidative stress can also lead to cardiovascular disease and hypercholesterolaemia, it has been shown that using Caveolin-1 can reduce the symptoms of hypercholesterolaemia and its effects, and this is due to the significant reduction of ROMO1 and ROMO1-derived ROS. Therefore, it can be concluded that ROMO1 can also be involved in the development of cardiovascular disease and hypercholesterolaemia.51 Obstructive Sleep Apnea Syndrome (OSAS) introduces repetitive incidents of temporary reduction and disrupts the oxygen supply, which leads to apneas and hypopneas among sleeping. When repetitive intermittent hypoxia and oxygen desaturation occur during sleep, some metabolic changes are triggered in the body, causing a loss of balance in the oxidant and antioxidant system, which ultimately increases oxidative stress and inflammation. Previous studies have shown that OSAS can cause various disorders such as systemic arterial hypertension, coronary artery disease, stroke and diabetes. Studies have shown that the mitochondrial ROMO1 protein can be a potential contributor to the development and progression of OSAS. Since it has been shown that ROMO1 has increased dramatically in the serum level of patients with OSAS, and introduced the protein as an important factor in the pathogenesis of disease, as well as offered ROMO1 for screening this respiratory disease.52 As stated, ROMO1 can engage in various activities in cells, and in relation to different signaling pathways, but an interesting feature in relation to this mitochondrial protein is its anti-bacterial property. Sha et al.,53 showed that ROMO1 could kill Escherichia coli BL-21 by penetrating the bacterial membrane. This anti-bacterial ROMO1 property is due to a special part of its structure called pCM19 peptide, corresponding to amino acids 42 to 60. This region has given antibacterial functionality to this protein.
By using a direct injection of pCM19 peptide into the body of the mice, Sha et al.53 found that the peptide could kill various bacteria, especially multi drug resistant (MDR) bacteria, with an efficiency of 100%. The interesting thing about this peptide was the lack of side impressions such as hemolysis, inside and outside of the condition of physiology of the body. So, it is hoped that ROMO1 can be used as an alternative drug in place of some antibiotics, specially MDR for killing bacteria, in the future.53 As has been mentioned earlier, ROMO1 can be involved in the production of ROS and their transfer to cytosol, and ROMO1-derived ROS is required for the growth of natural cells and also in cancerous cells. As we know, if ROMO1-induced ROS is produced too much and cannot be controlled, it can contribute to aging by damaging DNA. For this reason, in addition to the various effects of ROMO1 in the cell and on other molecules, the increase of ROMO1 expression can be related to the aging process.54
As we know, cancer in the world is increasing, and the most important causes are the increase of free radicals, but the causes of cancers are multi-factorial and also include inheritance and genetic mutations, environmental factors such as diet, stress, physical activity, lifestyle, exposure to radiation, etc. However, when cancer occurs, it should be identified at an early stage to lower costs and shorten recovery time. For this reason, a number of tumor markers in clinical laboratories are used to diagnose and identify a neoplasm. However, according to the information mentioned above, this important mitochondrial protein (ROMO1) can have features to be used in diagnostic laboratories, as a tumor marker. Not only can this protein be used to diagnose some cancers, but it can also be used as a therapeutic option. Since ROMO1 is associated with the level of oxidative stress and the production of ROS in cancerous cells, and by modulating its expression, it can reduce the symptoms of cancer and responds better to chemotherapy, because one of the most important causes for the incidence of cancer is the increase of free radicals and ROS. In addition, as mentioned, ROMO1 can have antibiotic properties and can be used in microbiology and infectious diseases in future. However, for a molecule to be used as a marker or drug, more detailed and wider research is needed, and more research is needed for ROMO1.
Figures and Tables
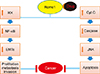 | FIG. 1This figure shows two important pathways that ROMO1 can play in the onset and progression of cancer. As we can see, it is possible to control this important protein using a Modulator, drug or everything and ultimately, with the signaling pathways, we can counteract with onset and progression of neoplasm. |
References
2. World Health Organization, Research for International Tobacco Control. WHO report on the global tobacco epidemic, 2008: the MPOWER package [Internet]. Geneva: World Health Organization;c2008. cited 2019 Apr 10. Available from: https://www.who.int/tobacco/mpower.
3. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.

4. Nowell PC. Mechanisms of tumor progression. Cancer Res. 1986; 46:2203–2207.
5. Pecorino L. Molecular biology of cancer: mechanisms, targets and therapeutics. 3rd ed. Oxford: Oxford University Press;2012. p. 66–70.
6. Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005; 65:10946–10951.

7. Nankali M, Karimi J, Goodarzi MT, Saidijam M, Khodadadi I, Razavi AN, et al. Increased expression of the receptor for advanced glycation end-products (RAGE) is associated with advanced breast cancer stage. Oncol Res Treat. 2016; 39:622–628.

8. Rahimi F, Karimi J, Goodarzi MT, Saidijam M, Khodadadi I, Razavi AN, et al. Overexpression of receptor for advanced glycation end products (RAGE) in ovarian cancer. Cancer Biomark. 2017; 18:61–68.

9. Moradi MN, Karimi J, Khodadadi I, Amiri I, Karami M, Saidijam M, et al. Evaluation of the p53 and Thioredoxin reductase in sperm from asthenozoospermic males in comparison to normozoospermic males. Free Radic Biol Med. 2018; 116:123–128.

10. Moridi H, Karimi J, Sheikh N, Goodarzi MT, Saidijam M, Yadegarazari R, et al. Resveratrol-dependent down-regulation of receptor for advanced glycation end-products and oxidative stress in kidney of rats with diabetes. Int J Endocrinol Metab. 2015; 13:e23542.

11. Lee S, Park YH, Chung JS, Yoo YD. ROMO1 and the NF-κB pathway are involved in oxidative stress-induced tumor cell invasion. Int J Oncol. 2015; 46:2021–2028.

12. Lin S, Li Y, Zamyatnin AA Jr, Werner J, Bazhin AV. Reactive oxygen species and colorectal cancer. J Cell Physiol. 2018; 233:5119–5132.

13. Tavilani H, Nadi E, Karimi J, Goodarzi MT. Oxidative stress in COPD patients, smokers, and non-smokers. Respir Care. 2012; 57:2090–2094.
14. Wu R, Feng J, Yang Y, Dai C, Lu A, Li J, et al. Significance of serum total oxidant/antioxidant status in patients with colorectal cancer. PLoS One. 2017; 12:e0170003.

15. Lee GY, You DG, Lee HR, Hwang SW, Lee CJ, Yoo YD. ROMO1 is a mitochondrial nonselective cation channel with viroporin-like characteristics. J Cell Biol. 2018; 217:2059–2071.

16. Na AR, Chung YM, Lee SB, Park SH, Lee MS, Yoo YD. A critical role for ROMO1-derived ROS in cell proliferation. Biochem Biophys Res Commun. 2008; 369:672–678.

17. Liu D, Liu Y, Xia Z, Dong H, Yi Z. Reactive oxygen species modulator 1 regulates oxidative stress and induces renal and pulmonary fibrosis in a unilateral ureteral obstruction rat model and in HK-2 cells. Mol Med Rep. 2017; 16:4855–4862.

18. Kim IG, Kim SY, Kim HA, Kim JY, Lee JH, Choi SI, et al. Disturbance of DKK1 level is partly involved in survival of lung cancer cells via regulation of ROMO1 and γ-radiation sensitivity. Biochem Biophys Res Commun. 2014; 443:49–55.

19. Setyawan EMN, Kim MJ, Oh HJ, Kim GA, Jo YK, Lee SH, et al. Spermine reduces reactive oxygen species levels and decreases cryocapacitation in canine sperm cryopreservation. Biochem Biophys Res Commun. 2016; 479:927–932.

20. Ranjbaran J, Farimani M, Tavilani H, Ghorbani M, Karimi J, Poormonsefi F, et al. Matrix metalloproteinases 2 and 9 and MMP9/NGAL complex activity in women with PCOS. Reproduction. 2016; 151:305–311.

21. Chung JS, Park S, Park SH, Park ER, Cha PH, Kim BY, et al. Overexpression of ROMO1 promotes production of reactive oxygen species and invasiveness of hepatic tumor cells. Gastroenterology. 2012; 143:1084–1094.e7.

22. Shyamsunder P, Verma RS, Lyakhovich A. ROMO1 regulates RedOx states and serves as an inducer of NF-κB-driven EMT factors in Fanconi anemia. Cancer Lett. 2015; 361:33–38.

23. Lee SB, Kim JJ, Chung JS, Lee MS, Lee KH, Kim BS, et al. ROMO1 is a negative-feedback regulator of Myc. J Cell Sci. 2011; 124(Pt 11):1911–1924.

24. Anand R, Wai T, Baker MJ, Kladt N, Schauss AC, Rugarli E, et al. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J Cell Biol. 2014; 204:919–929.

25. Kaser M, Kambacheld M, Kisters-Woike B, Langer T. Oma1, a novel membrane-bound metallopeptidase in mitochondria with activities overlapping with the m-AAA protease. J Biol Chem. 2003; 278:46414–46423.

26. Richter F, Dennerlein S, Nikolov M, Jans DC, Naumenko N, Aich A, et al. ROMO1 is a constituent of the human presequence translocase required for YME1L protease import. J Cell Biol. 2019; 218:598–614.

28. Kim JJ, Lee SB, Park JK, Yoo YD. TNF-alpha-induced ROS production triggering apoptosis is directly linked to Romo1 and Bcl-X(L). Cell Death Differ. 2010; 17:1420–1434.

29. Lee J, Kim SS. The function of p27 KIP1 during tumor development. Exp Mol Med. 2009; 41:765–771.
30. Chung JS, Lee SB, Park SH, Kang ST, Na AR, Chang TS, et al. Mitochondrial reactive oxygen species originating from ROMO1 exert an important role in normal cell cycle progression by regulating p27(Kip1) expression. Free Radic Res. 2009; 43:729–737.

31. Lee SB, Kim JJ, Kim TW, Kim BS, Lee MS, Yoo YD. Serum deprivation-induced reactive oxygen species production is mediated by ROMO1. Apoptosis. 2010; 15:204–218.

32. Yu MO, Song NH, Park KJ, Park DH, Kim SH, Chae YS, et al. ROMO1 is associated with ROS production and cellular growth in human gliomas. J Neurooncol. 2015; 121:73–81.

33. Mazzio EA, Boukli N, Rivera N, Soliman KF. Pericellular pH. homeostasis is a primary function of the Warburg effect: inversion of metabolic systems to control lactate steady state in tumor cells. Cancer Sci. 2012; 103:422–432.

34. Norton M, Ng AC, Baird S, Dumoulin A, Shutt T, Mah N, et al. ROMO1 is an essential redox-dependent regulator of mitochondrial dynamics. Sci Signal. 2014; 7:ra10.

35. Zhu Y, Yang Y, Li F, Fan S, Chen X, Lu Y, et al. Stimulation of the class-A scavenger receptor induces neutrophil extracellular traps (NETs) by ERK dependent NOX2 and ROMO1 activation. Biochem Biophys Res Commun. 2019; 511:847–854.

36. Kim HJ, Jo MJ, Kim BR, Kim JL, Jeong YA, Na YJ, et al. Reactive oxygen species modulator-1 (ROMO1) predicts unfavorable prognosis in colorectal cancer patients. PLoS One. 2017; 12:e0176834.

37. Chung YM, Kim JS, Yoo YD. A novel protein, ROMO1, induces ROS production in the mitochondria. Biochem Biophys Res Commun. 2006; 347:649–655.

38. Hwang IT, Chung YM, Kim JJ, Chung JS, Kim BS, Kim HJ, et al. Drug resistance to 5-FU linked to reactive oxygen species modulator 1. Biochem Biophys Res Commun. 2007; 359:304–310.

39. Lee SH, Lee JS, Lee EJ, Min KH, Hur GY, Lee SH, et al. Serum reactive oxygen species modulator 1 (ROMO1) as a potential diagnostic biomarker for non-small cell lung cancer. Lung Cancer. 2014; 85:175–181.

40. Chen X, Zhang N, Dong J, Sun G. Reactive oxygen species modulator 1, a novel protein, combined with carcinoembryonic antigen in differentiating malignant from benign pleural effusion. Tumour Biol. 2017; 39:1010428317698378.

41. Lee SH, Choi SI, Lee JS, Kim CH, Jung WJ, Lee EJ, et al. Reactive oxygen species modulator 1 (ROMO1) predicts poor outcomes in advanced non-small cell lung cancer patients treated with platinum-based chemotherapy. Cancer Res Treat. 2017; 49:141–149.

42. Wu H, Gu YH, Wei L, Guo TK, Zhao Y, Su G, et al. Association of ROMO1 gene genetic polymorphisms with risk of gastric cancer in northwestern chinese population. Pathol Oncol Res. 2015; 21:581–587.

43. Shin JA, Chung JS, Cho SH, Kim HJ, Yoo YD. ROMO1 expression contributes to oxidative stress-induced death of lung epithelial cells. Biochem Biophys Res Commun. 2013; 439:315–320.

44. Abdillah DA, Setyawan EMN, Oh HJ, Ra K, Lee SH, Kim MJ, et al. Iodixanol supplementation during sperm cryopreservation improves protamine level and reduces reactive oxygen species of canine sperm. J Vet Sci. 2019; 20:79–86.

45. John AMSP, Kundu S, Pushpakumar S, Fordham M, Weber G, Mukhopadhyay M, et al. GYY4137, a hydrogen sulfide donor modulates mir194-dependent collagen realignment in diabetic kidney. Sci Rep. 2017; 7:10924.

46. Petrovic MG, Kruzliak P, Petrovic D. The rs6060566 of the reactive oxygen species modulator 1 (ROMO-1) gene affects ROMO-1 expression and the development of diabetic retinopathy in Caucasians with type 2 diabetes. Acta Ophthalmol. 2015; 93:e654–e657.

47. Lin WC, Lee MT, Chang SC, Chang YL, Shih CH, Yu B, et al. Effects of mulberry leaves on production performance and the potential modulation of antioxidative status in laying hens. Poult Sci. 2017; 96:1191–1203.

48. Frank PG, Lisanti MP. Caveolin-1 and caveolae in atherosclerosis: differential roles in fatty streak formation and neointimal hyperplasia. Curr Opin Lipidol. 2004; 15:523–529.

49. Pelkmans L. Secrets of caveolae- and lipid raft-mediated endocytosis revealed by mammalian viruses. Biochim Biophys Acta. 2005; 1746:295–304.

50. Nixon SJ, Carter A, Wegner J, Ferguson C, Floetenmeyer M, Riches J, et al. Caveolin-1 is required for lateral line neuromast and notochord development. J Cell Sci. 2007; 120(Pt 13):2151–2161.

51. Chen YH, Lin WW, Liu CS, Hsu LS, Lin YM, Su SL. Caveolin-1 provides palliation for adverse hepatic reactions in hypercholesterolemic rabbits. PLoS One. 2014; 9:e71862.

52. Ye L, Qian Y, Li Q, Fang S, Yang Z, Tan Y, et al. Serum ROMO1 is significantly associated with disease severity in patients with obstructive sleep apnea syndrome. Sleep Breath. 2018; 22:743–748.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download