Abstract
Most differentiated thyroid cancer (DTC) patients have an excellent prognosis. However, about one-third of DTC patients with recurrent or metastatic disease lose the hallmark of specific iodine uptake initially or gradually and acquire radioactive iodine-refractory DTC (RAIR-DTC) with poor prognosis. Due to the potentially severe complications from unnecessarily repeated RAI therapy and encouraging progress of multiple targeted drugs for advanced RAIR-DTC patients, it has become crucial to identify RAIR-DTC early. In this review, we focus on the progress and controversies regarding the defining of RAIR-DTC, further with subsistent approaches and promising molecular nuclear medicine imaging in identifying RAIR-DTC, which may shed light on the proper management methodsof such patients.
The incidence of thyroid cancer has increased rapidly. 567,233 new cases of thyroid cancer occurred globally in 2018, accounting for 3.1% of all tumors.1 Differentiated thyroid cancer (DTC), including papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), Hürthle cell carcinoma, and poorly differentiated thyroid cancer (PDTC), arise from follicular cells and make up more than 95% of all of thyroid cancers.2 Irrespective of excellent prognoses in most DTC patients, the prevalence of distant metastasis ranges widely from 5%–25%,34567 which leads to a 5-year survival rate that can be as high as approximately 50%.8 However, about one-third of DTC patients with recurrence or metastasis lose the hallmark of specific iodine uptake initially or gradually, presenting as a RAI-refractory state.9 The 5-year survival rate was merely 19% for such patients.9 Inadequate response to RAI poses a great challenge RAI-refractory DTC (RAIR-DTC) management.
According to the 2015 American Thyroid Association (ATA) guidelines, the RAI therapy should be discontinued once the patient has been recognized as RAIR-DTC. Although the recognition of RAIR-DTC has been more and more clear over time, the diagnostic criteria are still based on imaging manifestation and RAI response, which is more or less influenced by the physician's objective judgment. There isn't a well-recognized definition of RAIR-DTC. Furthermore, along with the encouraging progress of molecular pathogenesis over the recent decades, multiple drugs targeted on genetic and epigenetic alterations, and aberrant signal pathways, notably tyrosine-kinase inhibitors (TKIs), have been developed with promising results and have begun to meaningfully impact clinical practice. With the advent of these new treatment options, practitioners are faced with important decisions in determining which patients are appropriate for systemic treatments and the proper timing to initiate the treatment. Thus, it has become crucial to early identify and even predict RAIR-DTC. Herein, we aim to address RAIR-DTC as follows: 1) the definition of and controversies surrounding RAIR-DTC; 2) subsistent approaches to recognize RAIR-DTC; and 3) promising molecular nuclear medicine imaging in identifying RAIR-DTC.
Paterson et al.10 reported the phenomenon of non-RAI avidity as early as 1952. However, no explicit statement had been clearly defined about RAIR-DTC until September 2010 during the 14th International Thyroid Congress in Paris, France, an international panel defined RAIR-DTC as a combination of at least one lesion which does not show RAI uptake on a RAI whole-body scan (WBS) or clinical evidence that RAI no longer provides a benefit to the patient.11 In September 2012 in Pisa, RAIR-DTC was complementally considered as one or more metastatic lesions failing to take up RAI and continuing to grow.12 Tuttle et al.13 detailed factors associated with suboptimal RAI avidity in 2014: a negative post-treatment WBS (RxWBS) after a properly administered RAI therapy, structural disease progression or rising serum thyroglobulin (Tg) 6–12 months after previous RAI therapy. In 2015, Sacks and Braunstein14 further proposed that the lack of RAI avidity in one or more lesions even using diagnostic 131I-WBS (DxWBS) was considered RAI-refractory disease. Subsequently, the 2015 ATA guidelines classified RAIR-DTC in the following basic ways: 1) when malignant/metastatic tissue does not concentrate RAI (no uptake outside the thyroid bed at the first RxWBS), 2) the tumor tissue loses the ability to concentrate RAI after previous evidence of RAI-avid disease (in the absence of stable iodine contamination), 3) RAI is concentrated in some lesions but not in others; and 4) metastatic disease progresses despite a significant concentration of RAI.15
Although the definition of RAIR-DTC has been developed over time, some controversies remain, particularly as to its clinical implication. Meanwhile, it is noteworthy that novel perspectives have been put forward recently.
The first controversy is the debate over the value of negative 131I-WBS imaging in RAIR-DTC proof. Negative 131I-WBS could be a fundamental indicator for RAIR-DTC identification, while it may not be a gold standard. Plenty of factors affect the reliability of 131I-WBS, including low-iodine preparation, prescribed activity, 131I-WBS acquisition timing, imaging techniques, RAI isotopes, etc. Furthermore, it has been claimed that the RAI activity of DxWBS might not detect all of the RAI-avid lesions. As many as 25% to 80% of patients with negative DxWBS were spotted with RAI-avid foci by RxWBS.16 While relevant studies suggested limited benefit from additional RAI treatments in patients with negative DxWBS but positive RxWBS.1718 Moreover, the interval between 131I-WBS acquisition and RAI administration could impact the detection of RAI-avid metastases.192021 Either earlier or later scans after administration of a 131I therapy may falsely classify RAI-avid metastasis as non-RAI-avid.22 Concerning imaging techniques, the superiority of PET to deliver images for detecting RAI-avid disease have been demonstrated compared with planar and SPECT.2324 Similarly, 124I showed a superior ability to detect RAI-avid lesions than 131I did. Notably, growing evidence has suggested that the RAI-avid metastatic lesions spotted on a 124I scan wouldn't guarantee more effective 131I treatment.2526
The second debate among nuclear medicine physicians is over patients with multiple metastases displaying imaging heterogeneity, which means avid and no-avid RAI metastases coexistence in one DTC patients. As it has been suggested in such cases, a combined strategy toward heterogeneous foci seems more reasonable, for example, a local treatment for RAI-refractory lesions (e.g. surgery, radiotherapy, radiofrequency ablation, etc.) and 131I therapy for RAI-avid tumors.27 Nevertheless, it has been questioned whether it really provide precise benefits for such patients. The potential risk of thyrotropin (TSH) stimulation to non-RAI-avid lesions during 131I therapy preparation is also cause for concern. Meanwhile, the probable contribution of the tumor burden by certain non-RAI-avid or RAI-avid lesions should be taken into account when tailoring the subsequent RAI therapy. Thus, the rationality of 131I therapy should be further evaluated and balanced in terms of benefit and risk.
Concerning the cumulative activity of RAI therapy, it may not be rational to take 22.2 GBq (600mCi) as a cut-off value of RAIR-DTC's definition. Given the increased risk of secondary cancers and leukemias with RAI activity accretion, patients with a cumulative dose of more than 22.2 GBq are not recommended for RAI therapy and classified as RAIR-DTC.928 However, it is noticeable that parts of such patients are still with visible RAI uptake in lesions which have neither been cured nor progress according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria, which might be regarded as a kind of response as “stable disease”. In cases like these, it is debatable whether additional RAI treatment should be recommended for such patients. The 2015 ATA guidelines suggest that subsequent RAI treatment should be based on the meaningful response, which is “generally associated with a significant reduction in serum Tg and/or in the size or rate of growth of metastases or structurally apparent disease”.15 Therefore, how to define response to RAI therapy is more crucial in such a scenario, rather than the argument of a specific cut-off value of RAI dosages.
Furthermore, novel perspectives about defining RAIR-DTC have been put forward as follows. 1) Patients with disease progressing whereas complementary total thyroidectomy is no longer beneficial merely for demonstrating RAIR-DTC by 131I-WBS. Such patients could be identified as RAIR-DTC by alternative molecular imaging other than 131I-WBS. 2) Benefits from RAI therapy in aggressive DTC histology (such as poorly differentiated, insular or Huürthle Cell variants).29 There is no sufficient evidence to determine whether RAI is likely to be effective in such a situation. Significant uptake of RAI and benefits still could be seen in up to 20% of patients with either Hürthle cell carcinoma or poorly differentiated phenotypes. Conversely, RAIR-DTC would occur even in classic PTC. Therefore, it is tough to judge the benefits of RAI therapy in patients with aggressive histology variants. Further evaluation remains necessary to determine whether it is refractory to iodine indeed. Given the unclearness in defining RAIR-DTC, it is not easy to confirm the RAI-refractory status. Further evaluation and more neutral approaches should be tailored to identify RAIR-DTC.
Due to the expression of sthe odium iodide symporter (NIS), the unique property of thyroid follicular cells to trap and concentrate iodine was also reserved in DTC, which allows RAI (generally 131I) to be an effective agent of imaging and therapy of DTC and its metastases. 131I-WBS can localize to the remnant thyroid issue and residual or recurrent RAI-avid lesions and provide evidence for subsequent 131I treatment. The accuracy of a DxWBS can reach up to 90%, especially with high specificity (91%–100%) and relatively low sensitivity (27–55%).30313233 Nevertheless, the neoplastic cells may dedifferentiate and lose certain characteristics associated with normal thyroid follicular cells, notably the diminished NIS expression and/or intracellular retention. About one-third of DTC patients with recurrence or metastases show negative 131I uptake initially or gradually.9 According to Durante et al.9 and Song et al.,34 patients without 131I uptake in their metastases presented significantly higher disease-specific mortality and drastically decreased 10-year survival rate, compared with those with 131I uptake. In addition, one retrospective analysis indicated that 131I uptake grade of metastatic disease was an independent prognostic factor.35 Adequate attention needs to be paid to patients with negative imaging of 131I-WBS (despite DxWBS or RxWBS) and abnormally elevated serum Tg level, which always suggests the presence of RAI-refractory disease (Fig. 1).
Because of the rare iodine avidity of RAIR-DTC foci, research interest into other effective imaging modalities has been aroused by the time. 18F-FDG, the most well-known functional radiotracer, also plays a crucial role in RAIR-DTC management, including the foci detection, efficacy evaluation and prognosis prediction (Fig. 1). Feine et al.36 first reported an inverse relationship between RAI and FDG uptake in thyroid carcinoma (the so-called ‘flip-flop phenomenon’), which was thought to be attributed to the loss of ability to concentrate RAI during dedifferentiation, along with an increased demand for glucose of tumor cells. In a meta-analysis of the diagnostic accuracy of 18F-FDG PET/CT in DTC patients with elevated serum Tg after thyroidectomy and negative 131I-WBS, the pooled sensitivity and specificity of 18F-FDG PET/CT were 93.5% (95% confidence interval [CI], 87.0%–97.3%) and 83.9% (95% CI, 72.3%–92.0%), respectively.37 There has been a variety of reports demonstrated that the sensitivity of 18F-FDG PET/CT findings increased with the Tg level.3839404142 18F-FDG PET metabolic parameters have been confirmed to be prognostic factors in several studies.43444546 In a study of 62 metastatic RAIR-DTC patients, Manohar et al.43 found that patients with overall metabolic tumor volumes (MTV)s higher than 9.08 mL and total lesion glycolysis (TLG) higher than 49.1 had poorer overall survival (OS) (p=0.06) and progression-free survival (PFS) (p=0.007) rates. According to the 2015 ATA guidelines, 18F-FDG PET/CT was recommended for high-risk DTC patients with elevated Tg levels (generally stimulated Tg>10 ng/mL) and negative RAI imaging.15 A recent prospective study showed that at a cut-off value of 4.0 in SUVmax, the sensitivity and specificity of 18F-FDG PET/CT predicting 131I-avidity could reach to 75.3% and 56.7%, respectively, which indicates that 18F-FDG PET/CT before 131I therapy is of great value in the prediction of the RAI-avidity of metastases.47
Tg is a protein produced by thyroid follicular cells. Under the condition of negative anti-Tg antibody, Tg is an important additional parameter in the determination of remission and monitoring of the disease's progress. The increasing trend or very high Tg levels after total thyroid ablation may suggest a recurrence and/or metastasis of thedisease. From a retrospective study of 137 patients with PTC after total thyroidectomy, Miyauchi et al.48 found that Tg-doubling time (Tg-DT) was a prominent independent predictor of prognosis. Patients with Tg-DT shorter than 1 year showed a 10-year-survival rate of 50%, which is significantly less than 95% of those with Tg-DT for 1–3 years. Yet, one study aiming at progressive or recurrent DTC patients drew a different conclusion that Tg-DT alone was not an independent survival predictor, but instead, that the highly significant difference in survival rates was revealed in patients with high tumor loads (Tg>100 ng/mL).49 The discrepancies between these studies might result from the different patient constitution and cohort comparison. Another assessment of Tg trends provides a brand-new perspective on Tg dynamic evaluation. It analyzed the ratio of the pre-ablative, stimulated Tg in the first 2 RAI therapies (pstim-Tg2/Tg1) and the ratio of suppressed Tg before and after the second RAI therapy (sup-Tg2/Tg1) from pulmonary metastatic DTC patients, which indicated that the higher Tg2/Tg1 value above the cutoff point (0.544 for pstim-Tg2/Tg1, 0.972 for sup-Tg2/Tg1), the greater possibility of RAIR-DTC.50
It is well known that the BRAFV600E mutation is correlated with the recurrence and poor clinicopathological outcomes.515253 The BRAFV600E mutation negatively regulates iodine metabolism genes (NIS, TSHR, Tg, TPO, etc) via the abnormal activation of the mitogen-activated protein kinase (MAPK) pathway.545556 On the one hand, BRAFV600E mutation promotes NIS silence by histone deacetylation (HDAC) of NIS promoter,57 on the other hand, the BRAFV600E mutation upgrades the secretion of transforming growth factor β (TGFβ), which, through the activation of SMADs (the family of intracellular transducers that act downstream of receptors for TGFβ family members) and sequential impairment of the thyroid-gene transcription factor PAX8, is a potent repressor of NIS in thyroid cells (Fig. 2).5859 Our previous study found that the BRAFV600E mutation was associated with the non-RAI-avid of DTC lesions in distant metastases.60 Similarly, the TERT mutation also closely associates with non-RAI-avid DTC within distant metastases, it offers a greater negative influence on RAI avidity when compared with the BRAFV600E mutation. Meanwhile, the TERT mutation could be used as an early predictor RAIR status.61 Several studies have further found that coexistence of the TERT and BRAFV600E mutations may trigger more aggressive clinicopathologic characteristics.626364656667
18F-FDG PET/CT is usually considered one of the most important alternative imaging strategies, which is still unable to detect all of the foci. Regarding further studies, refreshing specific radiotracer uptake showed potential benefits for identifying RAIR-DTC. The BRAF oncoprotein can activate ERK1/2 signal through the MAPK pathway, then thrombospondin-1 (TSP-1) is upregulated. TSP-1 positively regulates the expression of a cell membrane receptor, integrin, which in turn increases the level of ERK1/2 signal, forming positive feedback (Fig. 2).68 Therefore, Integrin can be a potential imaging target for the estimation of tumor angiogenesis. Integrin αvβ3 has been proposed as a molecular marker for radiolabeled RGD peptides. Our previous study showed that all the targeted RAIR-DTC metastatic lesions were identified as positive on 99mTc-3PRGD2 SPECT images in 10 DTC Patients, which provided hopeful imaging for the localization and growth evaluation of RAIR-DTC lesions.69 In one prospective research study, 81.1% of patients showed positive 99mTc -3PRGD2 uptake by SPECT/CT in 37 DTC patients who had negative 131I-WBS, but elevated Tg levels. The sensitivity and positive predictive value (PPV) were respectively 96.6% and 93.3% and increased with growing serum Tg levels.70 68Ga- DOTA-RGD PET imaging has higher sensitivity and resolution than 18F-FDG PET, which is more beneficial to the quantitative analysis of the lesions.71 Additionally, Chernaya et al. reported different expression levels of integrin receptors and their ligands in different DTC subtypes and could be affected by the BRAF mutation, which indicated that it is possible to more accurately select RGD imaging under individual clinical and pathological conditions.72
The prostate-specific membrane antigen (PSMA) is a new target for radionuclide imaging and therapy of prostate cancer in recent years.7374 Overexpression of PSMA has also been demonstrated on the cell membrane of endothelial cells of tumor neovasculature in several other malignancies such as renal cell carcinoma, colon carcinoma, neuroendocrine tumors, melanoma, and breast cancer.7576 It is compelling that 68Ga-PSMA PET/CT imaging can also identify RAIR-DTC potentially. In a prospective research study including 10 patients of metastatic DTC harboring 32 lesions, all patients showed substantial PSMA uptake with 30/32 total lesions detected by 68Ga-PSMA PET, compared 23/32 positive lesions on 18F-FDG PET/CT. Particularly, 21 (70%) of the 30 lesions showing PSMA expression were localized to the bones.77 Verburg et al.78 provided imaging evidence of PSMA expression in DTC using 68Ga PSMA-HBED-CC PET/CT. Recently in a study by Lütje et al.,79 6 patients with 131I-WBS-negative and 18F-FDG-positive metastasized DTC received 68Ga -labeled PSMA ligands and underwent PET/CT. The results demonstrated that 68Ga-HBED-CC-PSMA PET/CT might be suitable for staging patients with RAI-negative DTC metastases and identifying patients who might be eligible for PSMA-targeted radionuclide therapy.
Numerous studies have demonstrated the expression of somatostatin receptors (SSTR) type 2, 3 and 5 in DTC in variable percentages.80818283 Radiolabeled somatostatin analogs, such as 68Ga-DOTA-octreotide and 68Ga-DOTA-lanreotide (LAN), have drawn worldwide attention because of their superior pharmacokinetic characteristics and better spatial resolution of PET technology.848586 However, the diagnostic value of radiolabeled somatostatin analogues in RAIR-DTC remains conflicting. In a study by Traub-Weidinger et al.,87 lesions showing aerobic glycolysis on 18F-FDG PET were found in 24 (86%) of 28 patients with corresponding positive results with 68Ga-DOTA-LAN in 35% and with 68Ga-DOTA-Tyr3-octreotide in 29%. Kundu et al.88 also confirmed 68Ga-DOTA-NOC PET-CT is inferior to 18F-FDG PET-CT at the lesion-based level in DTC with raised Tg and negative 131I-WBS.
The role of radiolabeled choline PET/CT has been fully verified in the diagnosis of prostate cancer. Incidental thyroid uptake has been reported by authors in 18F-choline PET/CT prostate cancer scintigraphy. Some researchers have reported that radiolabeled choline PET/CT may be useful in hunting metastases of thyroid cancer which were negative on 18F-FDG PET/CT. Wu et al.89 successfully identified thyroid carcinoma using 11C-choline PET/CT in 4 patients with thyroid carcinomas, while the lesions in 3 of 4 patients were missed by previous 18F-FDG PET. In a case report by Piccardo et al.,90 it concluded that 18F-choline PET/CT could detect lethal DTC recurrences, thus choline PET/CT may complement 18F-FDG PET/CT in identifying DTC lesions. Given the absence of convincing evidence, the effectiveness of 11C-choline imaging needs to be further verified with large-scale research (Fig. 3).
The management of RAIR-DTC has been a huge challenge for clinical physicians. There are still many controversies on the definition of RAIR-DTC. The current advancements in RAIR-DTC diagnosis are limited to post-131I therapy evaluation, in which the patients may have been exposed to unnecessary RAI radiation and missed the opportunity to receive more effective interventions. There is a need to be able to predict RAIR-DTC before 131I therapy and make individualized treatment decisions. Thus, continuous improvement in molecular imaging and molecular pathology are surely needed in future research and should focus on RAI-refractory prediction, treatment targets selection, determining the optimal timing for treatment initiation and making second and even third-line treatment schedules.
Figures and Tables
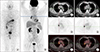 | FIG. 1A 52-year-old man with DTC with negative 131I-WBS and positive 18F-FDG PET/CT. The patient had an elevated serum Tg but negative 131I-WBS. 18F-FDG PET/CT was recommended and displayed two right hilar lymph nodes with metastatic carcinoma. (A) 131I-WBS, (B) PET MIP imaging, (C, F) CT, (D, G) PET, (E, H) fusion. |
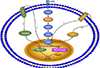 | FIG. 2The mechanism of BRAFV600E mutation negatively regulates sodium iodide symporter (NIS) and Integrin targeted imaging. On the one hand, BRAFV600E mutation promotes NIS silence by histone deacetylation (HDAC) of NIS promoter, on the other hand, BRAFV600E mutation promotes the secretion of transforming growth factor β (TGFβ), which, through the activation of SMADs and consequent impairment of the thyroid-gene transcription factor PAX8. BRAF oncoprotein can activate ERK1/2 signal through mitogen-activated protein kinase (MAPK) pathway, then thrombospondin-1 (TSP-1) was upregulated, TSP-1 further positively regulates the expression of integrin, which in turn increases the level of ERK1/2 signal, forming a positive feedback. |
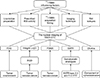 | FIG. 3The nuclear imaging for identifying RAIR-DTC. Negative 131I-WBS act as fundamental indicator for RAIR-DTC identifying, while there still are plenty of influencing factors. Other nuclear imaging modalities could be complements with progressed researches. 131I-WBS: 131I-whole-body scan, RAI: radioactive iodine, RAIR-DTC: radioactive iodine refractory differentiated thyroid cancer, FDG: fluorodeoxyglucose, RGD: Arg-Gly-Asp, PSMA: prostate-specific membrane antigen, SSTR: Somatostatin receptors. |
ACKNOWLEDGEMENTS
This research was supported by the National Natural Sciences Foundation of China (81771875 and 81571714), Medical and Health Science and Technology Innovation Project of Chinese Academy of Medical Science in 2018 (2016-I2M-2-006), and part from the Thyroid Study Group of the Asia Oceania Research Initiative Network (AORIN).
Notes
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.

2. Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al. SEER cancer statistics review, 1975–2013 [Internet]. Bethesda: National Cancer Institue;c2016. cited 2019 May 15. Available from: http://seer.cancer.gov/csr/1975_2013.
3. Sampson E, Brierley JD, Le LW, Rotstein L, Tsang RW. Clinical management and outcome of papillary and follicular (differentiated) thyroid cancer presenting with distant metastasis at diagnosis. Cancer. 2007; 110:1451–1456.

4. Schlumberger M, Tubiana M, De Vathaire F, Hill C, Gardet P, Travagli JP, et al. Long-term results of treatment of 283 patients with lung and bone metastases from differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1986; 63:960–967.

5. Albano D, Bertagna F, Bonacina M, Durmo R, Cerudelli E, Gazzilli M, et al. Possible delayed diagnosis and treatment of metastatic differentiated thyroid cancer by adopting the 2015 ATA guidelines. Eur J Endocrinol. 2018; 179:143–151.

6. Haq M, Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome. Clin Endocrinol (Oxf). 2005; 63:87–93.

7. Albano D, Panarotto MB, Durmo R, Rodella C, Bertagna F, Giubbini R. Clinical and prognostic role of detection timing of distant metastases in patients with differentiated thyroid cancer. Endocrine. 2019; 63:79–86.

8. Nixon IJ, Whitcher MM, Palmer FL, Tuttle RM, Shaha AR, Shah JP, et al. The impact of distant metastases at presentation on prognosis in patients with differentiated carcinoma of the thyroid gland. Thyroid. 2012; 22:884–889.

9. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006; 91:2892–2899.

10. Paterson R, Warrington HC, Gilbert CW. Radioiodine in thyroid cancer. Br Med Bull. 1952; 8:154–157.

11. Brose MS, Smit J, Capdevila J, Elisei R, Nutting C, Pitoia F, et al. Regional approaches to the management of patients with advanced, radioactive iodine-refractory differentiated thyroid carcinoma. Expert Rev Anticancer Ther. 2012; 12:1137–1147.

12. Schlumberger M, Brose M, Elisei R, Leboulleux S, Luster M, Pitoia F, et al. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014; 2:356–358.

13. Tuttle RM, Sabra MM. Defining RAI refractory thyroid cancer: when is RAI therapy unlikely to achieve a therapeutic response?[Internet]. South Dartmouth: Thyroid Disease Manager;c2014. cited 2019 Jul 9. Available from: https://www.thyroidmanager.org/wp-content/uploads/chapters/s2-defining-rai-refractory-thyroid-cancer-when-is-rai-therapy-unlikely-to-achieve-a-therapeutic-response.pdf.
14. Sacks W, Braunstein GD. Evolving approaches in managing radioactive iodine-refractory differentiated thyroid cancer. Endocr Pract. 2014; 20:263–275.

15. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.

16. Wells K, Moreau S, Shin YR, Van Nostrand D, Burman K, Wartofsky L. Positive (+) post-treatment (tx) scans after the radioiodine (RAI) tx of patients who have well-differentiated thyroid cancer (WDTC), positive serum thyroglobulin levels (TG+), and negative diagnostic (dx) RAI whole body scans (WBS−): predictive values and frequency. J Nucl Med. 2008; 49:Suppl 1. 238P.
17. Sabra MM, Grewal RK, Tala H, Larson SM, Tuttle RM. Clinical outcomes following empiric radioiodine therapy in patients with structurally identifiable metastatic follicular cell-derived thyroid carcinoma with negative diagnostic but positive post-therapy 131I whole-body scans. Thyroid. 2012; 22:877–883.

18. Fatourechi V, Hay ID, Javedan H, Wiseman GA, Mullan BP, Gorman CA. Lack of impact of radioiodine therapy in tg-positive, diagnostic whole-body scan-negative patients with follicular cell-derived thyroid cancer. J Clin Endocrinol Metab. 2002; 87:1521–1526.

19. Hung BT, Huang SH, Huang YE, Wang PW. Appropriate time for post-therapeutic I-131 whole body scan. Clin Nucl Med. 2009; 34:339–342.

20. Lee JW, Lee SM, Koh GP, Lee DH. The comparison of (131)I whole-body scans on the third and tenth day after (131)I therapy in patients with well-differentiated thyroid cancer: preliminary report. Ann Nucl Med. 2011; 25:439–446.

21. Chong A, Song HC, Min JJ, Jeong SY, Ha JM, Kim J, et al. Improved detection of lung or bone metastases with an I-131 whole body scan on the 7th day after high-dose I-131 therapy in patients with thyroid cancer. Nucl Med Mol Imaging. 2010; 44:273–281.

22. Salvatori M, Perotti G, Villani MF, Mazza R, Maussier ML, Indovina L, et al. Determining the appropriate time of execution of an I-131 post-therapy whole-body scan: comparison between early and late imaging. Nucl Med Commun. 2013; 34:900–908.

23. Phan HT, Jager PL, Paans AM, Plukker JT, Sturkenboom MG, Sluiter WJ, et al. The diagnostic value of 124I-PET in patients with differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008; 35:958–965.

24. Beijst C, Kist JW, Elschot M, Viergever MA, Hoekstra OS, de Keizer B, et al. Quantitative comparison of 124I PET/CT and 131I SPECT/CT detectability. J Nucl Med. 2016; 57:103–108.

25. Ruhlmann M, Jentzen W, Ruhlmann V, Pettinato C, Rossi G, Binse I, et al. High level of agreement between pretherapeutic 124I PET and intratherapeutic 131I imaging in detecting iodine-positive thyroid cancer metastases. J Nucl Med. 2016; 57:1339–1342.

26. Kist JW, de Keizer B, van der Vlies M, Brouwers AH, Huysmans DA, van der Zant FM, et al. 124I PET/CT to predict the outcome of blind 131I treatment in patients with biochemical recurrence of differentiated thyroid cancer: results of a multicenter diagnostic cohort study (THYROPET). J Nucl Med. 2016; 57:701–707.

27. Tuttle RM, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH, et al. Controversies, consensus, and collaboration in the use of (131)I therapy in differentiated thyroid cancer: a joint statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid. 2019; 29:461–470.

28. Rubino C, de Vathaire F, Dottorini ME, Hall P, Schvartz C, Couette JE, et al. Second primary malignancies in thyroid cancer patients. Br J Cancer. 2003; 89:1638–1644.

29. Riesco-Eizaguirre G, Galofré JC, Grande E, Zafón Llopis C, Ramón y Cajal Asensio T, Navarro González E, et al. Spanish consensus for the management of patients with advanced radioactive iodine refractory differentiated thyroid cancer. Endocrinol Nutr. 2016; 63:e17–e24.

30. Lubin E, Mechlis-Frish S, Zatz S, Shimoni A, Segal K, Avraham A, et al. Serum thyroglobulin and iodine-131 whole-body scan in the diagnosis and assessment of treatment for metastatic differentiated thyroid carcinoma. J Nucl Med. 1994; 35:257–262.
31. Franceschi M, Kusić Z, Franceschi D, Lukinac L, Roncević S. Thyroglobulin determination, neck ultrasonography and iodine-131 whole-body scintigraphy in differentiated thyroid carcinoma. J Nucl Med. 1996; 37:446–451.
32. Filesi M, Signore A, Ventroni G, Melacrinis FF, Ronga G. Role of initial iodine-131 whole-body scan and serum thyroglobulin in differentiated thyroid carcinoma metastases. J Nucl Med. 1998; 39:1542–1546.
33. Mazzaferri EL, Kloos RT. Is diagnostic iodine-131 scanning with recombinant human TSH useful in the follow-up of differentiated thyroid cancer after thyroid ablation? J Clin Endocrinol Metab. 2002; 87:1490–1498.

34. Song HJ, Qiu ZL, Shen CT, Wei WJ, Luo QY. Pulmonary metastases in differentiated thyroid cancer: efficacy of radioiodine therapy and prognostic factors. Eur J Endocrinol. 2015; 173:399–408.

35. Higashi T, Nishii R, Yamada S, Nakamoto Y, Ishizu K, Kawase S, et al. Delayed initial radioactive iodine therapy resulted in poor survival in patients with metastatic differentiated thyroid carcinoma: a retrospective statistical analysis of 198 cases. J Nucl Med. 2011; 52:683–689.

36. Feine U, Lietzenmayer R, Hanke JP, Held J, Wöhrle H, Müller-Schauenburg W. Fluorine-18-FDG and iodine-131-iodide uptake in thyroid cancer. J Nucl Med. 1996; 37:1468–1472.
37. Dong MJ, Liu ZF, Zhao K, Ruan LX, Wang GL, Yang SY, et al. Value of 18F-FDG-PET/PET-CT in differentiated thyroid carcinoma with radioiodine-negative whole-body scan: a meta-analysis. Nucl Med Commun. 2009; 30:639–650.

38. Trybek T, Kowalska A, Lesiak J, Młynarczyk J. The role of 18F-Fluorodeoxyglucose Positron Emission Tomography in patients with suspected recurrence or metastatic differentiated thyroid carcinoma with elevated serum thyroglobulin and negative I-131 whole body scan. Nucl Med Rev Cent East Eur. 2014; 17:87–93.

39. Ozkan E, Aras G, Kucuk NO. Correlation of 18F-FDG PET/CT findings with histopathological results in differentiated thyroid cancer patients who have increased thyroglobulin or antithyroglobulin antibody levels and negative 131I whole-body scan results. Clin Nucl Med. 2013; 38:326–331.

40. Kwon SY, Kim J, Jung SH, Chong A, Song HC, Bom HS, et al. Preablative stimulated thyroglobulin levels can predict malignant potential and therapeutic responsiveness of subcentimetersized, 18F-fluorodeoxyglucose-avid cervical lymph nodes in patients with papillary thyroid cancer. Clin Nucl Med. 2016; 41:e32–e38.

41. Stangierski A, Kaznowski J, Wolinski K, Jodlowska E, Michaliszyn P, Kubiak K, et al. The usefulness of fluorine-18 fluorodeoxyglucose PET in the detection of recurrence in patients with differentiated thyroid cancer with elevated thyroglobulin and negative radioiodine whole-body scan. Nucl Med Commun. 2016; 37:935–938.

42. Vera P, Kuhn-Lansoy C, Edet-Sanson A, Hapdey S, Modzelewski R, Hitzel A, et al. Does recombinant human thyrotropin-stimulated positron emission tomography with [18F]fluoro-2-deoxy-D-glucose improve detection of recurrence of well-differentiated thyroid carcinoma in patients with low serum thyroglobulin? Thyroid. 2010; 20:15–23.

43. Manohar PM, Beesley LJ, Bellile EL, Worden FP, Avram AM. Prognostic value of FDG-PET/CT metabolic parameters in metastatic radioiodine-refractory differentiated thyroid cancer. Clin Nucl Med. 2018; 43:641–647.

44. Masson-Deshayes S, Schvartz C, Dalban C, Guendouzen S, Pochart JM, Dalac A, et al. Prognostic value of (18)F-FDG PET/CT metabolic parameters in metastatic differentiated thyroid cancers. Clin Nucl Med. 2015; 40:469–475.

45. Pace L, Klain M, Salvatore B, Nicolai E, Zampella E, Assante R, et al. Prognostic role of 18F-FDG PET/CT in the postoperative evaluation of differentiated thyroid cancer patients. Clin Nucl Med. 2015; 40:111–115.

46. Robbins RJ, Wan Q, Grewal RK, Reibke R, Gonen M, Strauss HW, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab. 2006; 91:498–505.

47. Liu M, Cheng L, Jin Y, Ruan M, Sheng S, Chen L. Predicting (131)I-avidity of metastases from differentiated thyroid cancer using (18)F-FDG PET/CT in postoperative patients with elevated thyroglobulin. Sci Rep. 2018; 8:4352.

48. Miyauchi A, Kudo T, Miya A, Kobayashi K, Ito Y, Takamura Y, et al. Prognostic impact of serum thyroglobulin doubling-time under thyrotropin suppression in patients with papillary thyroid carcinoma who underwent total thyroidectomy. Thyroid. 2011; 21:707–716.

49. Rössing RM, Jentzen W, Nagarajah J, Bockisch A, Görges R. Serum thyroglobulin doubling time in progressive thyroid cancer. Thyroid. 2016; 26:1712–1718.

50. Wang C, Zhang X, Li H, Li X, Lin Y. Quantitative thyroglobulin response to radioactive iodine treatment in predicting radioactive iodine-refractory thyroid cancer with pulmonary metastasis. PLoS One. 2017; 12:e0179664.

51. Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015; 33:42–50.

52. Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013; 309:1493–1501.

53. Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007; 28:742–762.

54. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013; 13:184–199.

56. Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005; 90:6373–6379.

57. Zhang Z, Liu D, Murugan AK, Liu Z, Xing M. Histone deacetylation of NIS promoter underlies BRAF V600E-promoted NIS silencing in thyroid cancer. Endocr Relat Cancer. 2014; 21:161–173.

58. Riesco-Eizaguirre G, Rodríguez I, De la Vieja A, Costamagna E, Carrasco N, Nistal M, et al. The BRAFV600E oncogene induces transforming growth factor beta secretion leading to sodium iodide symporter repression and increased malignancy in thyroid cancer. Cancer Res. 2009; 69:8317–8325.

59. Costamagna E, García B, Santisteban P. The functional interaction between the paired domain transcription factor Pax8 and Smad3 is involved in transforming growth factor-beta repression of the sodium/iodide symporter gene. J Biol Chem. 2004; 279:3439–3446.

60. Yang K, Wang H, Liang Z, Liang J, Li F, Lin Y. BRAFV600E mutation associated with non-radioiodine-avid status in distant metastatic papillary thyroid carcinoma. Clin Nucl Med. 2014; 39:675–679.

61. Yang X, Li J, Li X, Liang Z, Gao W, Liang J, et al. TERT promoter mutation predicts radioiodine-refractory character in distant metastatic differentiated thyroid cancer. J Nucl Med. 2017; 58:258–265.

62. Liu X, Qu S, Liu R, Sheng C, Shi X, Zhu G, et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J Clin Endocrinol Metab. 2014; 99:E1130–E1136.
63. Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014; 32:2718–2726.

64. Ngeow J, Eng C. TERT and BRAF in thyroid cancer: teaming up for trouble. J Clin Oncol. 2014; 32:2683–2684.

65. Melo M, da Rocha AG, Vinagre J, Sobrinho-Simões M, Soares P. Coexistence of TERT promoter and BRAF mutations in papillary thyroid carcinoma: added value in patient prognosis? J Clin Oncol. 2015; 33:667–668.

66. Dettmer MS, Schmitt A, Steinert H, Capper D, Moch H, Komminoth P, et al. Tall cell papillary thyroid carcinoma: new diagnostic criteria and mutations in BRAF and TERT. Endocr Relat Cancer. 2015; 22:419–429.

67. Moon S, Song YS, Kim YA, Lim JA, Cho SW, Moon JH, et al. Effects of coexistent BRAF(V600E) and TERT promoter mutations on poor clinical outcomes in papillary thyroid cancer: a meta-analysis. Thyroid. 2017; 27:651–660.

68. Nucera C, Lawler J, Parangi S. BRAF(V600E) and microenvironment in thyroid cancer: a functional link to drive cancer progression. Cancer Res. 2011; 71:2417–2422.

69. Zhao D, Jin X, Li F, Liang J, Lin Y. Integrin αvβ3 imaging of radioactive iodine-refractory thyroid cancer using 99mTc-3PRGD2. J Nucl Med. 2012; 53:1872–1877.

70. Gao R, Zhang GJ, Wang YB, Liu Y, Wang F, Jia X, et al. Clinical value of 99mTc-3PRGD2 SPECT/CT in differentiated thyroid carcinoma with negative 131I whole-body scan and elevated thyroglobulin level. Sci Rep. 2018; 8:473.

71. Vatsa R, Shykla J, Mittal BR, Bhusari P, Sood A, Basher RK, et al. Usefulness of 68Ga-DOTA-RGD (αvβ3) PET/CT imaging in thyroglobulin elevation with negative iodine scintigraphy. Clin Nucl Med. 2017; 42:471–472.

72. Chernaya G, Mikhno N, Khabalova T, Svyatchenko S, Mostovich L, Shevchenko S, et al. The expression profile of integrin receptors and osteopontin in thyroid malignancies varies depending on the tumor progression rate and presence of BRAF V600E mutation. Surg Oncol. 2018; 27:702–708.

73. Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015; 42:197–209.

74. Kratochwil C, Giesel FL, Eder M, Afshar-Oromieh A, Benešová M, Mier W, et al. [177Lu]Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2015; 42:987–988.

75. Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999; 59:3192–3198.
76. Demirci E, Ocak M, Kabasakal L, Decristoforo C, Talat Z, Halaç M, et al. (68)Ga-PSMA PET/CT imaging of metastatic clear cell renal cell carcinoma. Eur J Nucl Med Mol Imaging. 2014; 41:1461–1462.

77. Verma P, Malhotra G, Agrawal R, Sonavane S, Meshram V, Asopa RV. Evidence of prostate-specific membrane antigen expression in metastatic differentiated thyroid cancer using 68Ga-PSMA-HBED-CC PET/CT. Clin Nucl Med. 2018; 43:e265–e268.

78. Verburg FA, Krohn T, Heinzel A, Mottaghy FM, Behrendt FF. First evidence of PSMA expression in differentiated thyroid cancer using [68Ga]PSMA-HBED-CC PET/CT. Eur J Nucl Med Mol Imaging. 2015; 42:1622–1623.

79. Lütje S, Gomez B, Cohnen J, Umutlu L, Gotthardt M, Poeppel TD, et al. Imaging of prostate-specific membrane antigen expression in metastatic differentiated thyroid cancer using 68Ga-HBEDCC-PSMA PET/CT. Clin Nucl Med. 2017; 42:20–25.

80. Teunissen JJ, Kwekkeboom DJ, Kooij PP, Bakker WH, Krenning EP. Peptide receptor radionuclide therapy for non-radioiodineavid differentiated thyroid carcinoma. J Nucl Med. 2005; 46 Suppl 1:107S–114S.
81. Klagge A, Krause K, Schierle K, Steinert F, Dralle H, Fuhrer D. Somatostatin receptor subtype expression in human thyroid tumours. Horm Metab Res. 2010; 42:237–240.

82. Pisarek H, Stepień T, Kubiak R, Borkowska E, Pawlikowski M. Expression of somatostatin receptor subtypes in human thyroid tumors: the immunohistochemical and molecular biology (RT-PCR) investigation. Thyroid Res. 2009; 2:1.

83. Sancak S, Hardt A, Singer J, Klöppel G, Eren FT, Güllüoglu BM, et al. Somatostatin receptor 2 expression determined by immunohistochemistry in cold thyroid nodules exceeds that of hot thyroid nodules, papillary thyroid carcinoma, and Graves' disease. Thyroid. 2010; 20:505–511.

84. Stokkel MP, Verkooijen RB, Smit JW. Indium-111 octreotide scintigraphy for the detection of non-functioning metastases from differentiated thyroid cancer: diagnostic and prognostic value. Eur J Nucl Med Mol Imaging. 2004; 31:950–957.

85. Virgolini I, Patri P, Novotny C, Traub T, Leimer M, Füger B, et al. Comparative somatostatin receptor scintigraphy using in-111-DOTA-lanreotide and in-111-DOTA-Tyr3-octreotide versus F-18-FDG-PET for evaluation of somatostatin receptor-mediated radionuclide therapy. Ann Oncol. 2001; 12 Suppl 2:S41–S45.

86. Rodrigues M, Traub-Weidinger T, Leimer M, Li S, Andreae F, Angelberger P, et al. Value of 111In-DOTA-lanreotide and 111In-DOTA-DPhe1-Tyr3-octreotide in differentiated thyroid cancer: results of in vitro binding studies and in vivo comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2005; 32:1144–1151.

87. Traub-Weidinger T, Putzer D, von Guggenberg E, Dobrozemsky G, Nilica B, Kendler D, et al. Multiparametric PET imaging in thyroid malignancy characterizing tumour heterogeneity: somatostatin receptors and glucose metabolism. Eur J Nucl Med Mol Imaging. 2015; 42:1995–2001.

88. Kundu P, Lata S, Sharma P, Singh H, Malhotra A, Bal C. Prospective evaluation of (68)Ga-DOTANOC PET-CT in differentiated thyroid cancer patients with raised thyroglobulin and negative (131)I-whole body scan: comparison with (18)F-FDG PET-CT. Eur J Nucl Med Mol Imaging. 2014; 41:1354–1362.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download