1. Kudo H, Kuwamura K, Izawa I, Sawa H, Tamaki N. Chronic subdural hematoma in elderly people: present status on Awaji Island and epidemiological prospect. Neurol Med Chir (Tokyo). 1992; 32:207–209.

2. Stippler M, Ramirez P, Berti A, Macindoe C, Villalobos N, Murray-Krezan C. Chronic subdural hematoma patients aged 90 years and older. Neurol Res. 2013; 35:243–246.

3. Almenawer SA, Farrokhyar F, Hong C, Alhazzani W, Manoranjan B, Yarascavitch B, Arjmand P, Baronia B, Reddy K, Murty N, Singh S. Chronic subdural hematoma management: a systematic review and meta-analysis of 34,829 patients. Ann Surg. 2014; 259:449–457.
4. Kim Y, Lim SH, Park GY. Crossed cerebellar diaschisis has an adverse effect on functional outcome in the subacute rehabilitation phase of stroke: a case-control study. Arch Phys Med Rehabil. 2019; 100:1308–1316.

5. Yoo YJ, Kim JW, Kim JS, Hong BY, Lee KB, Lim SH. Corticospinal tract integrity and long-term hand function prognosis in patients with stroke. Front Neurol. 2019; 10:374.

6. Miranda LB, Braxton E, Hobbs J, Quigley MR. Chronic subdural hematoma in the elderly: not a benign disease. J Neurosurg. 2011; 114:72–76.

7. Mulligan P, Raore B, Liu S, Olson JJ. Neurological and functional outcomes of subdural hematoma evacuation in patients over 70 years of age. J Neurosci Rural Pract. 2013; 4:250–256.

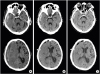
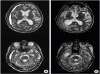
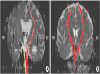
8. Yokoyama K, Matsuki M, Shimano H, Sumioka S, Ikenaga T, Hanabusa K, Yasuda S, Inoue H, Watanabe T, Miyashita M, Hiramatsu R, Murao K, Kondo A, Tanabe H, Kuroiwa T. Diffusion tensor imaging in chronic subdural hematoma: correlation between clinical signs and fractional anisotropy in the pyramidal tract. AJNR Am J Neuroradiol. 2008; 29:1159–1163.

9. Sawauchi S, Beaumont A, Signoretti S, Tomita Y, Dunbar J, Marmarou A. Diffuse brain injury complicated by acute subdural hematoma and secondary insults in the rodents: the effect of surgical evacuation. Acta Neurochir Suppl (Wien). 2002; 81:241–242.

10. Yokobori S, Nakae R, Yokota H, Spurlock MS, Mondello S, Gajavelli S, Bullock RM. Subdural hematoma decompression model: a model of traumatic brain injury with ischemic-reperfusional pathophysiology: a review of the literature. Behav Brain Res. 2018; 340:23–28.

11. Cho SH, Kim DG, Kim DS, Kim YH, Lee CH, Jang SH. Motor outcome according to the integrity of the corticospinal tract determined by diffusion tensor tractography in the early stage of corona radiata infarct. Neurosci Lett. 2007; 426:123–127.

12. Rosso C, Valabregue R, Attal Y, Vargas P, Gaudron M, Baronnet F, Bertasi E, Humbert F, Peskine A, Perlbarg V, Benali H, Lehéricy S, Samson Y. Contribution of corticospinal tract and functional connectivity in hand motor impairment after stroke. PLoS One. 2013; 8:e73164.

13. Seo JP, Do KH, Jung GS, Seo SW, Kim K, Son SM, Kim YK, Jang SH. The difference of gait pattern according to the state of the corticospinal tract in chronic hemiparetic stroke patients. NeuroRehabilitation. 2014; 34:259–266.

14. Shin HE, Suh HC, Kang SH, Seo KM, Kim DK, Shin HW. Diagnostic challenge of diffusion tensor imaging in a patient with hemiplegia after traumatic brain injury. Ann Rehabil Med. 2017; 41:153–157.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download