This article has been
cited by other articles in ScienceCentral.
Abstract
Lingual dystonia is a rare type of dystonia, the main symptom of which varies from intermittent to sustained tongue fixation. Several studies have suggested that the cerebellum may be implicated in dystonia. There are several treatment options available for dystonia, including medication, botulinum toxin injection, and surgical intervention. We chose to inject botulinum toxin into the styloglossus muscle, and the symptoms of the lingual dystonia were improved. We report a case of lingual dystonia following a bilateral cerebellar stroke that responded to treatment with botulinum toxin.
Highlights
• Lingual dystonia is a type of dystonia, which involved focally in tongue muscle.
• Cerebellum could be associated with lingual dystonia.
• Lingual dystonia was improved through botulinum toxin injection.
Keywords: Dystonia, Cerebellum, Stroke, Botulinum Toxins
INTRODUCTION
Dystonia is a syndrome characterized by sustained muscle contractions, which frequently induce repetitive and twisting movements or abnormal postures [
1]. Oromandibular dystonia can be involved in masticatory, facial, and cervical muscles, however, lingual dystonia is involved focally in tongue muscle. Lingual dystonia can be either primary or secondary. It is known to be related to brain damage, neuroleptic use, neurodegenerative, metabolic, and neurodevelopmental disorders, and virus infection [
2]. It has a negative impact on many daily activities, including speaking, chewing, and swallowing, and it also causes social and vocational disabilities [
3].
The basal ganglia have traditionally been thought to be associated with dystonia. Yet, several studies have also suggested the association of the cerebellum and its connections with dystonia [
45].
There are several treatment options available for dystonia, including pharmacological therapies, botulinum toxin injection, and surgical intervention such as deep brain stimulation. Pharmacological therapies represent the first choice of treatment for generalized dystonia, while botulinum toxin injection is preferred in cases of focal or segmental dystonia [
6].
Here, we report on the case of a patient who developed lingual dystonia following a bilateral cerebellar stroke. The patient's symptoms were improved after the injection of botulinum toxin into the styloglossus muscle, without any clinical complications.
CASE REPORT
A 49-year-old man with a history of chronic alcoholism visited our emergency room due to dizziness, gait disturbance, and dysarthria. Brain magnetic resonance imaging (MRI) was performed, which showed recent infarctions in the bilateral cerebellar hemispheres (
Fig. 1) and no other findings were seen in the other areas. The patient was thus admitted to the neurology department.
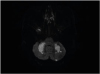 | Fig. 1 Diffusion-weighted magnetic resonance imaging of the patient showing recent infarctions in the bilateral cerebellar hemispheres.
|
The neurological examination revealed an intact gag reflex, full range of eye movement, nystagmus in both eyes, and ataxia in both upper and lower extremities. Additionally, he also displayed dysarthria, difficulty swallowing, and lingual dystonia accompanied by tongue tilting toward the palate and limited tongue movement. These movements were more pronounced during speech and swallowing.
The Korean version of the western aphasia battery conducted upon admission showed an Aphasia Quotient of 24.2/100. The patient could hear and understand the words, and obey a three-step command. However, in terms of language expression, the patient could only speak using vowels such as “
ah” and “
auh,” and he was not able to express consonants (
Supplementary Video 1).
At the time of admission, a videofluoroscopic swallowing study (VFSS) was also performed in order to evaluate the patient's difficulty swallowing. The VFSS showed the patient's tongue to be moving upwards involuntarily, accompanied by deteriorated tongue movement and posterior propelling of the oral phase (
Supplementary Video 2).
The patient exhibited several additional symptoms, including shivering, agitation, and visual and tactile hallucination. Hence, 0.5 mg of risperidone was administered due to the indications of delirium tremens which was caused by not drinking alcohol for several days.
We postulated that the cause of both the dysarthria and difficulty swallowing was lingual dystonia resulting from the brain lesion. Wilson's disease was excluded since the Kayser-Fleischer ring was not seen on the ophthalmologic examination. In addition, the patient had no history of psychiatric, hereditary metabolic or degenerative disease, and no specific developmental abnormality was seen prior to the onset of stroke. Additionally, the patient had never taken any antipsychotics or anticonvulsants. It is important to note here that risperidone was taken after dystonia occurred in this case.
Risperidone was tapered out completely, since it might be a cause of dystonia. Next, the patient was given 2 mg of trihexyphenidyl for the treatment of lingual dystonia, which did not result in an improvement in his symptoms, and he showed dizziness. Finally, the trihexyphenidyl was stopped. And then, total 50 units of botulinum toxin mixed in 0.5 mL normal saline were injected evenly into the bilateral styloglossus muscles. The examiner sitting in front of the patient. He placed the hockey stick transducer transversely on the patient's tongue and targeted styloglossus muscle with an in-plane technique. The injection sites were placed in the middle part of the tongue, and the number of motor points was 2 on each side. The injection was performed two months after the onset of dystonia.
Nine days after the injection of botulinum toxin, a follow-up VFSS was performed. Decreased dystonia was observed during the oral phase (
Supplementary Video 3). Further, the patient was able to pronounce sounds more clearly than before (
Supplementary Video 4).
Two years later, another follow-up VFSS was performed, which still showed a deterioration in tongue movement and posterior propelling. There was no additional injection in 2 years, but when compared to the previous test, the tongue dystonia with backward tongue movement was slightly improved.
DISCUSSION
Lingual dystonia could be caused by a variety of primary or secondary conditions [
1]. In this case, considering the patient's history and the onset following a cerebellar stroke, we were able to conclude that the lingual dystonia was associated with a brain lesion. The patient was taken risperidone. However, we could exclude risperidone as a cause of lingual dystonia, because the patient initially presented with dystonia prior to the addition of risperidone.
For decades, the basal ganglia were thought to be mainly associated with dystonia. Although the pathophysiology of dystonia has not been clarified, several case reports have indicated a relationship between the cerebellum and dystonia [
78]. In addition, imaging and molecular findings from recent studies have suggested that the cerebellum could also be associated [
5].
Several experimental animal studies have reported that abnormal signaling resulting from the cerebellum induces a dystonic symptom [
91011]. Normal mice displayed dystonic postures in response to kainic acid injection into the cerebellum. The severity of dystonia increased linearly with a dose of kainic acid [
9]. Also, the chronic application of kainic acid into the cerebellar vermis of rats caused a prolonged and generalized dystonic movement [
10]. Abnormalities in Purkinje cells were sufficient to induce dystonia by using conditional genetics to regionally limit cerebellar dysfunction. Moreover, the extent of dystonic movements was determined by the extent of cerebellar dysfunction [
11]. Through the above studies, the cerebellum, as well as the circuits between the cerebellum and basal ganglia, could play an important role in inducing dystonia.
As imaging techniques have been developed, researches related to the relationship between the cerebellum and dystonia have been reported [
121314]. The patients having focal hand dystonia showed decreased gray matter density in the sensorimotor territory of the cerebellum [
12]. And, the patients with idiopathic cervical dystonia showed increased gray matter density in the cerebellar flocculus as well as bilaterally in the motor cortex [
13]. The other research has shown lower resting functional connectivity in the cerebellum, thalamus, basal ganglia, bilateral supplementary motor area in the hand dystonia patients [
14]. In the future, as the functional MRI technique develops, subsequent studies should be conducted to determine which area including the cerebellum is associated with dystonia.
Treatment is also important because lingual dystonia causes discomfort on daily activities such as speaking, chewing and swallowing. Several prior studies have demonstrated that the injection of botulinum toxin into the tongue muscles can be a very effective and relatively safe treatment modality [
151617]. Moreover, it did not have any significant adverse effects, and the average duration of response to the botulinum toxin was reported to be 12.4 weeks [
3].
Considering the prior reports, we also performed a botulinum toxin injection. In many case reports, the genioglossus muscle was responsible for tongue protrusion, which makes it an ideal candidate for injection [
1617]. However, in this case, the tongue was tilting toward the palate, so we injected the botulinum toxin into the styloglossus muscle, which is the tongue flexor muscle. The dose of injected botulinum toxin was determined empirically. There were no adverse effects or complications following the injection of botulinum toxin.
In conclusion, this case report is meaningful in that lingual dystonia could occur after bilateral cerebellar stroke, and it was improved through botulinum toxin injection without complication. And, the important difference from the previous reports is that the target muscle of the injection was styloglossus muscle, not genioglossus muscle.



