This article has been
cited by other articles in ScienceCentral.
Abstract
Osteosarcoma is the most common primary bone tumor after plasma cell neoplasms. Osteosarcoma has diverse histological features and is characterized by the presence of malignant spindle cells and pluripotent neoplastic mesenchymal cells that produce immature bone, cartilage, and fibrous tissue. Osteosarcoma most frequently develops in the extremities of long bones, but can occur in the jaw in rare cases. The clinical and biological behavior of osteosarcoma of the jaw slightly differs from that of long-bone osteosarcoma. The incidence of jaw osteosarcoma is greater in the third to fourth decades of life, whereas long-bone osteosarcoma mostly occurs in the second decade of life. Osteosarcoma of the jaw has a lower tendency to metastasize and a better prognosis than long-bone osteosarcoma. Radiographically, osteosarcoma can present as a poorly-defined lytic, sclerotic, or mixed-density lesion with periosteal bone reaction response. Multi-detector computed tomography is useful for identifying the extent of bone destruction, as well as soft tissue involvement of the lesion. The current case report presents a fibroblastic osteosarcoma involving the left hemimandible with very unusual radiographic features.
Keywords: Osteosarcoma, Fibroblasts, Jaw, Radiography
Osteosarcoma is a malignancy of mesenchymal cells capable of producing osteoid, and it is the most common bone tumor after plasma cell neoplasms. Chondroid material and fibrous connective tissue may also be produced by the tumor. Histologically, osteosarcoma is classified into osteoblastic, chondroblastic, and fibroblastic subtypes.
1 In general, the differences in histologic classifications may not have an impact on the prognosis. However, fibroblastic differentiation has been found to be associated with a better prognosis and shows the best response to chemotherapy.
23 Osteosarcoma mostly develops in long bones, but approximately 5% of lesions occur in the jaws.
45 Osteosarcoma shows a bimodal age distribution, with the first peak in early adolescence and the second peak among older adults.
67
Osteosarcoma of the jaw is a rare malignant bone tumor that mostly develops in the posterior region of the mandible.
89 The peak incidence of osteosarcoma of the jaw is in the third to fourth decades of life, almost a decade later than peak incidence of long-bone osteosarcoma.
7 According to Unni and Dahlin's study,
10 osteosarcoma of the jaw has a better prognosis than conventional osteosarcoma. Histologically, the osteoblastic subtype constitutes the majority of jaw osteosarcomas, whereas the fibroblastic subtype rarely affects the jaws.
11
The clinical manifestation of osteosarcoma of the jaw includes swelling, pain, and paresthesia of the involved region.
9 Radiographically, the tumors appear as a poorly-defined lytic, sclerotic, or mixed-density lesion with a spiculated periosteal reaction.
11 Multi-detector computed tomography is useful for determining the degree of osseous destruction, and in most instances soft tissue involvement, in order to delineate the clinical stage and plan appropriate treatment.
11
This report presented a case of osteosarcoma of the mandible in a 31-year-old male Hispanic patient, with a unique radiographic and histologic appearance, which accordingly posed a challenge for diagnosis and effective management.
Case Report
A 31-year-old Hispanic man presented to the emergency clinic with a complaint of pain in the left mandible which had progressively worsened over the course of 2 weeks. The patient noted swelling in the past month with a rapid worsening of symptoms that prompted him to seek care. A clinical examination revealed an extraoral swelling with facial asymmetry of the left side of the face and paresthesia. Intraorally, floating left mandibular posterior teeth were noted. The patient denied any systemic complaints including fever, weakness, shortness of breath, and/or any history of primary cancer.
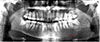
A panoramic image was acquired (Planmeca ProMax digital panoramic X-ray unit, Planmeca Oy, Helsinki, Finland) with exposure parameters of 70 kVp, 10 mA, and 16 s. An ill-defined, mostly lytic lesion involving the left side of the mandible was observed, extending approximately from the left mid-ramus toward the median mandible anteroposteriorly, and from the alveolar crest toward the inferior border of the mandible in the superoinferior direction. Thinning and disruption of the alveolar crest and inferior border of the mandible were noted (
Fig. 1).
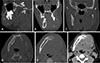
Multi-detector computed tomography was recommended for a better evaluation of the extent of the lesion and the involvement of adjacent anatomic entities, including soft tissue invasion. Multi-detector computed tomographic images revealed an osteolytic lesion within the left mandibular body, extending from the superior portion of the ramus toward the mandibular right first premolar region. The involved left molar and premolar teeth demonstrated a floating appearance. Non-uniform expansion with significant destruction of the cortices was noted, and the left inferior alveolar canal could not be traced. The left mental foramen was involved with the lesion. There was no evidence of a spiculated periosteal reaction. Scalloping of the margins was noted at different areas, which is typically noted in benign tumors. Left-side soft tissue swelling with an asymmetric nasopharyngeal airway was observed (
Fig. 2).
Based on the clinical findings, history of a tumor with rapid growth and neurologic deficits, and radiographic presentation, the differential diagnoses of malignant neoplasms such as squamous cell carcinoma and osteosarcoma were considered. However, due to the presence of expansion with scalloping borders, aggressive benign neoplasms such as desmoplastic fibroma and aggressive ameloblastoma were considered as well. The patient underwent an initial incisional biopsy, followed by histopathologic evaluation.
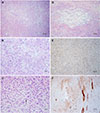
The incisal biopsy was returned with the suspected diagnosis of a spindle cell neoplasm. The neoplasm had perforated the cortical bone. The neoplasm was composed of spindle cells with round to elongated nuclei and indistinct cell borders. Some areas showed a storiform pattern (
Fig. 3A). Overall, the cell morphology was bland, with mitotic figures only being encountered occasionally (
Fig. 3B). No obvious osteoid formation was noted. Immunohistochemical stains for Ki-67, smooth muscle actin (SMA), and MDM2 were performed with valid positive controls. Fewer than 5% of the spindle cells of the lesion showed positivity for Ki-67. Scattered positivity for SMA and MDM2 was seen, but most of the neoplastic cells were negative for these 2 stains.
The diagnosis was spindle cell neoplasm, with comments on the differential diagnoses, including desmoplastic fibroma, low-grade fibrosarcoma, and low-grade fibroblastic osteosarcoma.
A segmental resection of the left body of the mandible was performed with wide surgical resection margins. Furthermore, the resection specimen showed a malignant spindle cell neoplasm in which the spindle cells were arranged in a fascicular pattern. Pleomorphism, numerous mitotic figures, and atypical mitoses could be identified in focal areas (
Fig. 3C). Osteoid formation was noted in several areas (
Fig. 3D). Extensive immunohistochemical studies were performed, including S100, SMA, MyoD1, myogenin, desmin, muscle-specific actin, SOX10, melanoma cocktail, CD34, SATB2, H3K27me3, and Ki-67. The tumor cells stained positive for SATB2 (
Fig. 3E) and H3K27me3. There was focal positivity for S100 and SMA (
Fig. 3F). The areas of SMA positivity were most prominent at the periphery of the tumor, near the periosteum or at the invasive front in the skeletal muscle. Ki-67 was found to be positive in 5%–15% of the neoplastic cells. The spindle cells were negative for all other stains. The final diagnosis was high-grade fibroblastic osteosarcoma.
Discussion
In osteosarcoma, which is the most common primary malignant lesion of the skeleton following hematopoietic neoplasms, malignant mesenchymal cells produce variable amounts of osteoid or immature bone.
1 This neoplasm represents approximately 20% of malignant bone tumors and can be divided into primary and secondary osteosarcoma. Primary osteosarcoma is classified as central (arising in the medullary cavity), surface (arising in the juxtacortical region), or, very rarely, extraskeletal (arising in the soft tissue).
12 Secondary osteosarcoma occurs in the setting of pre-existing bone abnormalities, such as Paget's disease of bone, fibrous dysplasia, multiple osteochondromas, chronic osteomyelitis, and osteogenesis imperfecta. Osteosarcoma can also arise in 2 cancer susceptibility syndromes: hereditary retinoblastoma and Li-Fraumeni syndrome.
12131415
Osteosarcomas develop most often in the long bones of the arms and legs, followed by the pelvis.
4 Osteosarcoma of the maxillofacial region, which is sometimes referred to as gnathic osteosarcoma, is generally a rare neoplasm, and accounts for approximately 5% to 13% of all osteosarcomas.
516 In the maxillofacial region, mandibular tumors occur more frequently in the posterior body, followed by the angle, ramus, and symphysis. Maxillary lesions arise more commonly in the alveolar ridge and maxillary antra.
28
Long-bone osteosarcomas occur in both the pediatric and adult population, with a major peak during adolescence coinciding with the pubertal growth spurt, and a second, but lesser peak among adults older than 65 years; usually secondary to malignant degeneration of Paget's disease or at the site of previous irradiation. Gnathic osteosarcoma occurs over a broad age range, with a mean age of 34–36 years, about 1 to 2 decades later than the mean age of incidence for osteosarcoma of the long bones. Gnathic osteosarcoma has a lower tendency for distant metastasis than long-bone osteosarcoma.
1718
The main symptoms of jaw osteosarcoma are localized pain and swelling, but paresthesia, loose teeth, and mucosal ulceration (usually not seen until late-stage disease) have been reported. Symptoms are usually present for several months, and it takes on average 3 to 4 months for a case to be diagnosed.
2819 Our case demonstrated a much more rapid progression.
According to the predominant tissue present microscopically, osteosarcoma is classified into osteoblastic, chondroblastic, and fibroblastic subtypes. Among these histologic subtypes, osteoblastic osteosarcoma accounts for the majority of jaw osteosarcomas, whereas fibroblastic osteosarcoma rarely affects the jaws.
11 In the present case, spindle cells were histologically predominant, and the immunohistochemical findings were most consistent with a high-grade fibroblastic osteosarcoma.
Radiographically, osteosarcoma shows a varied radiographic appearance, as it can present as a lytic, sclerotic, or mixed lytic-sclerotic lesion with ill-defined borders. Some osteosarcomas show a periosteal reaction manifesting as a sunburst pattern caused by radiating mineralized tumor spicules or a triangular elevation of the periosteum (Codman's triangle). Localized widening of the periodontal ligament space of 1 or 2 teeth in the absence of dental disease may occur in an early stage of osteosarcoma. Another feature of the neoplasm is destruction of the cortices with frank adjacent soft tissue invasion.
8111820 Biopsy is essential for confirming the diagnosis and establishing the histopathologic subtype of the osteosarcoma.
12
Conventional radiographs provide limited information in evaluating osteosarcomas due to the superimposed bony structures. However, the presence of widening of the periodontal ligament space and bone destruction in conventional radiographs warrant early detection of the lesion.
11 Contrast-enhanced multi-detector computed tomographic images show moderate enhancement of the solid component of the neoplasm. Bone-window multi-detector computed tomographic images show bone erosion and destruction clearly. Soft tissue-window multi-detector computed tomographic images are useful for the evaluation of spiculated periosteal response and soft tissue invasion of a neoplasm.
11 The present case showed an ill-defined lytic lesion with significant destruction of bone, with no classic sunburst pattern involving the peripheral bone. Multi-detector computed tomography findings ruled out any metastasis in the present case.
The radiographic differential diagnoses of osteosarcoma include osteomyelitis and chondrosarcoma.
720 Osteomyelitis may be of odontogenic or hematologic origin, appearing as a distinctly lytic bone signal with sequestration and associated periosteal reaction. However, the periosteal reaction is typically laminar, rather than spicules perpendicular to the cortex, as seen in osteosarcomas. Clinical features indicating the presence of an acute inflammatory response or long-standing changes may also be evident. When internal radiopacities are present in the tumor, chondrosarcoma must be considered as a differential diagnosis. However, chondrosarcomas can be slow-growing as well, mimicking a benign tumor.
720
Histopathologically, osteosarcoma may resemble a fibro-osseous lesion such as fibrous dysplasia. Immunohistochemical nuclear expression of the MDM2 and CDK4 proteins may help establish the diagnosis.
2021 Fibroblastic osteosarcomas show high-grade spindle cell stroma with focal areas of bone production. Spindle cell proliferation also seen in the fibrosarcoma, and can be confused with fibroblastic osteosarcoma. Occasionally, fibroblastic osteosarcoma may have pleomorphic nuclei and resemble malignant fibrous histiocytoma. However, the presence of even a small amount of osteoid matrix distinguishes fibroblastic osteosarcoma from the aforementioned neoplasms.
22 According to Sanerkin's study,
23 alkaline phosphatase was abundant in the malignant cells in osteosarcoma; however, this enzyme was present at low levels or absent entirely in fibrosarcoma.
Management of sarcomas involves a combination of wide surgical resection and chemotherapy, followed by periodic follow-up. Nuclear medicine imaging such as positron emission tomography is also carried out following computed tomography or magnetic resonance imaging to further assess the potential presence of metastatic lesions.
2425 In a previous study, fibroblastic osteosarcoma showed the best response to chemotherapy, whereas chondroblastic osteosar coma showed the poorest response.
26 In the case presented here, the patient was treated with a partial mandibulectomy and reconstruction with a titanium reconstruction plate coupled with chemotherapy.
In conclusion, osteosarcoma is a rare malignant bone tumor of the oral and maxillofacial region, which is enigmatic in many ways and still not completely understood. A comprehensive understanding of the radiographic appearance and histologic manifestations of osteosarcoma reduces the diagnostic difficulties in its identification and categorization, and also helps differentiate it from aggressive benign disease. The variation in the radiographic presentation of osteosarcoma must be borne in mind while reviewing imaging studies of patients with unusual imaging features and/or historical and clinical findings.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download