1. Cowburn AS, Sladek K, Soja J, Adamek L, Nizankowska E, Szczeklik A, et al. Overexpression of leukotriene C4 synthase in bronchial biopsies from patients with aspirin-intolerant asthma. J Clin Invest. 1998; 101:834–846.

2. Sampson AP, Cowburn AS, Sladek K, Adamek L, Nizankowska E, Szczeklik A, et al. Profound overexpression of leukotriene C4 synthase in bronchial biopsies from aspirin-intolerant asthmatic patients. Int Arch Allergy Immunol. 1997; 113:355–357.
3. Kim SH, Choi JH, Holloway JW, Suh CH, Nahm DH, Ha EH, et al. Leukotriene-related gene polymorphisms in patients with aspirin-intolerant urticaria and aspirin-intolerant asthma: differing contributions of ALOX5 polymorphism in Korean population. J Korean Med Sci. 2005; 20:926–931.

4. Kim SH, Bae JS, Suh CH, Nahm DH, Holloway JW, Park HS. Polymorphism of tandem repeat in promoter of 5-lipoxygenase in ASA-intolerant asthma: a positive association with airway hyperresponsiveness. Allergy. 2005; 60:760–765.

5. Sousa AR, Parikh A, Scadding G, Corrigan CJ, Lee TH. Leukotriene-receptor expression on nasal mucosal inflammatory cells in aspirin-sensitive rhinosinusitis. N Engl J Med. 2002; 347:1493–1499.

6. Arm JP, O'Hickey SP, Spur BW, Lee TH. Airway responsiveness to histamine and leukotriene E4 in subjects with aspirin-induced asthma. Am Rev Respir Dis. 1989; 140:148–153.
7. Kim SH, Oh JM, Kim YS, Palmer LJ, Suh CH, Nahm DH, et al. Cysteinyl leukotriene receptor 1 promoter polymorphism is associated with aspirin-intolerant asthma in males. Clin Exp Allergy. 2006; 36:433–439.

8. Akahoshi M, Obara K, Hirota T, Matsuda A, Hasegawa K, Takahashi N, et al. Functional promoter polymorphism in the TBX21 gene associated with aspirin-induced asthma. Hum Genet. 2005; 117:16–26.

9. Ying S, Meng Q, Scadding G, Parikh A, Corrigan CJ, Lee TH. Aspirin-sensitive rhinosinusitis is associated with reduced E-prostanoid 2 receptor expression on nasal mucosal inflammatory cells. J Allergy Clin Immunol. 2006; 117:312–318.

10. Pérez-Novo CA, Watelet JB, Claeys C, Van Cauwenberge P, Bachert C. Prostaglandin, leukotriene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J Allergy Clin Immunol. 2005; 115:1189–1196.

11. Schmid M, Göde U, Schäfer D, Wigand ME. Arachidonic acid metabolism in nasal tissue and peripheral blood cells in aspirin intolerant asthmatics. Acta Otolaryngol. 1999; 119:277–280.
12. Wang G, Zhang J, Sun H, Cao W, Zhang J, Wang Y, et al. Genetic variation in members of the leukotrienes biosynthesis pathway confers risk of ischemic stroke in Eastern Han Chinese. Prostaglandins Leukot Essent Fatty Acids. 2012; 87:169–175.

13. Wang GN, Zhang JS, Cao WJ, Sun H, Zhang J, Wang Y, et al. Association of ALOX5, LTA4H and LTC4S gene polymorphisms with ischemic stroke risk in a cohort of Chinese in east China. World J Emerg Med. 2013; 4:32–37.

14. Zhao N, Liu X, Wang Y, Liu X, Li J, Yu L, et al. Association of inflammatory gene polymorphisms with ischemic stroke in a Chinese Han population. J Neuroinflammation. 2012; 9:162.

15. Lima JJ, Zhang S, Grant A, Shao L, Tantisira KG, Allayee H, et al. Influence of leukotriene pathway polymorphisms on response to montelukast in asthma. Am J Respir Crit Care Med. 2006; 173:379–385.

16. Zhang Y, Huang H, Huang J, Xiang Z, Yang M, Tian C, et al. The −444A/C polymorphism in the LTC4S gene and the risk of asthma: a meta-analysis. Arch Med Res. 2012; 43:444–450.

17. Drazen JM, Yandava CN, Dubé L, Szczerback N, Hippensteel R, Pillari A, et al. Pharmacogenetic association between ALOX5 promoter genotype and the response to anti-asthma treatment. Nat Genet. 1999; 22:168–170.

18. Kim SH, Park HS. Genetic markers for differentiating aspirin-hypersensitivity. Yonsei Med J. 2006; 47:15–21.

19. Park SM, Park JS, Park HS, Park CS. Unraveling the genetic basis of aspirin hypersensitivity in asthma beyond arachidonate pathways. Allergy Asthma Immunol Res. 2013; 5:258–276.

20. Dahlin A, Weiss ST. Genetic and epigenetic components of aspirin-exacerbated respiratory disease. Immunol Allergy Clin North Am. 2016; 36:765–789.

21. Jenkins C, Costello J, Hodge L. Systematic review of prevalence of aspirin induced asthma and its implications for clinical practice. BMJ. 2004; 328:434.

22. Chang HS, Park JS, Jang AS, Park SW, Uh ST, Kim YH, et al. Diagnostic value of clinical parameters in the prediction of aspirin-exacerbated respiratory disease in asthma. Allergy Asthma Immunol Res. 2011; 3:256–264.

23. Moon JY, Kim SH, Kim TB, Kim SH, Chang YS, Lee JH, et al. Aspirin-intolerant asthma in the Korean population: prevalence and characteristics based on a questionnaire survey. Respir Med. 2013; 107:202–208.

24. Szczeklik A, Nizankowska E, Duplaga M. Natural history of aspirin-induced asthma. Eur Respir J. 2000; 16:432–436.

25. Bavbek S, Yilmaz I, Celik G, Aydin O, Erkekol FO, Orman A, et al. Prevalence of aspirin-exacerbated respiratory disease in patients with asthma in Turkey: a cross-sectional survey. Allergol Immunopathol (Madr). 2012; 40:225–230.

26. Rosado A, Vives R, González R, Rodríguez J. Can NSAIDs intolerance disappear? A study of three cases. Allergy. 2003; 58:689–690.

27. Lockey RF, Rucknagel DL, Vanselow NA. Familial occurrence of asthma, nasal polyps and aspirin intolerance. Ann Intern Med. 1973; 78:57–63.

28. Delaney JC. Letter: asthma, nasal polyposis, and aspirin sensitivity. Ann Intern Med. 1973; 79:761.
29. Settipane GA, Pudupakkam RK. Aspirin intolerance. III. Subtypes, familial occurence, and cross-reactivity with tartarazine. J Allergy Clin Immunol. 1975; 56:215–221.
30. Brasky TM, Bonner MR, Moysich KB, Ambrosone CB, Nie J, Tao MH, et al. Non-steroidal anti-inflammatory drug (NSAID) use and breast cancer risk in the Western New York Exposures and Breast Cancer (WEB) Study. Cancer Causes Control. 2010; 21:1503–1512.

31. Li X, Gao L, Cui Q, Gary BD, Dyess DL, Taylor W, et al. Sulindac inhibits tumor cell invasion by suppressing NF-κB-mediated transcription of microRNAs. Oncogene. 2012; 31:4979–4986.

32. Yiannakopoulou E. Modulation of lymphangiogenesis: a new target for aspirin and other nonsteroidal anti-inflammatory agents? A systematic review. J Clin Pharmacol. 2012; 52:1749–1754.

33. Pan MR, Chang HC, Chuang LY, Hung WC. The nonsteroidal anti-inflammatory drug NS398 reactivates SPARC expression via promoter demethylation to attenuate invasiveness of lung cancer cells. Exp Biol Med (Maywood). 2008; 233:456–462.

34. Tahara T, Shibata T, Nakamura M, Yamashita H, Yoshioka D, Okubo M, et al. Chronic aspirin use suppresses CDH1 methylation in human gastric mucosa. Dig Dis Sci. 2010; 55:54–59.

35. Cheong HS, Park SM, Kim MO, Park JS, Lee JY, Byun JY, et al. Genome-wide methylation profile of nasal polyps: relation to aspirin hypersensitivity in asthmatics. Allergy. 2011; 66:637–644.

36. Kim YJ, Park SW, Kim TH, Park JS, Cheong HS, Shin HD, et al. Genome-wide methylation profiling of the bronchial mucosa of asthmatics: relationship to atopy. BMC Med Genet. 2013; 14:39.

37. Tan L, Ou J, Tao Z, Kong Y, Xu Y. Neonatal immune state is influenced by maternal allergic rhinitis and associated with regulatory T cells. Allergy Asthma Immunol Res. 2017; 9:133–141.

38. Suh DI, Chang HY, Lee E, Yang SI, Hong SJ. Prenatal maternal distress and allergic diseases in offspring: review of evidence and possible pathways. Allergy Asthma Immunol Res. 2017; 9:200–211.

39. Tham EH, Leung DYM. How different parts of the world provide new insights into food allergy. Allergy Asthma Immunol Res. 2018; 10:290–299.

40. Zhou D, Li Z, Yu D, Wan L, Zhu Y, Lai M, et al. Polymorphisms involving gain or loss of CpG sites are significantly enriched in trait-associated SNPs. Oncotarget. 2015; 6:39995–40004.

41. Yoshida H, Nadanaka S, Sato R, Mori K. XBP1 is critical to protect cells from endoplasmic reticulum stress: evidence from Site-2 protease-deficient Chinese hamster ovary cells. Cell Struct Funct. 2006; 31:117–125.

42. Ono SJ, Liou HC, Davidon R, Strominger JL, Glimcher LH. Human X-box-binding protein 1 is required for the transcription of a subset of human class II major histocompatibility genes and forms a heterodimer with c-fos. Proc Natl Acad Sci U S A. 1991; 88:4309–4312.

43. Park BL, Kim TH, Kim JH, Bae JS, Pasaje CF, Cheong HS, et al. Genome-wide association study of aspirin-exacerbated respiratory disease in a Korean population. Hum Genet. 2013; 132:313–321.

44. Park JS, Son JH, Park CS, Chang HS. Clinical implications of single nucleotide polymorphisms in diagnosis of asthma and its subtypes. Yonsei Med J. 2019; 60:1–9.

45. Bochenek G, Nagraba K, Nizankowska E, Szczeklik A. A controlled study of 9alpha,11beta-PGF2 (a prostaglandin D2 metabolite) in plasma and urine of patients with bronchial asthma and healthy controls after aspirin challenge. J Allergy Clin Immunol. 2003; 111:743–749.
46. Lee JU, Chang HS, Lee HJ, Bae DJ, Son JH, Park JS, et al. Association of interleukin-25 levels with development of aspirin induced respiratory diseases. Respir Med. 2017; 123:71–78.

47. Yoshimura T, Yoshikawa M, Otori N, Haruna S, Moriyama H. Correlation between the prostaglandin D2/E2 ratio in nasal polyps and the recalcitrant pathophysiology of chronic rhinosinusitis associated with bronchial asthma. Allergol Int. 2008; 57:429–436.
48. Oh SH, Kim YH, Park SM, Cho SH, Park JS, Jang AS, et al. Association analysis of thromboxane A synthase 1 gene polymorphisms with aspirin intolerance in asthmatic patients. Pharmacogenomics. 2011; 12:351–363.

49. Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011; 31:986–1000.

50. Sanak M, Pierzchalska M, Bazan-Socha S, Szczeklik A. Enhanced expression of the leukotriene C4 synthase due to overactive transcription of an allelic variant associated with aspirin-intolerant asthma. Am J Respir Cell Mol Biol. 2000; 23:290–296.
51. Van Sambeek R, Stevenson DD, Baldasaro M, Lam BK, Zhao J, Yoshida S, et al. 5′ flanking region polymorphism of the gene encoding leukotriene C4 synthase does not correlate with the aspirin-intolerant asthma phenotype in the United States. J Allergy Clin Immunol. 2000; 106:72–76.

52. Cornejo-García JA, Perkins JR, Jurado-Escobar R, García-Martín E, Agúndez JA, Viguera E, et al. Pharmacogenomics of prostaglandin and leukotriene receptors. Front Pharmacol. 2016; 7:316.



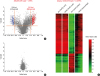





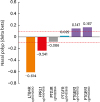





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download