Abstract
Since its introduction in 1995, uterine artery embolization (UAE) has become an established option for the treatment of leiomyomas. Identification of a leiomyoma using arteriography improves the ability to perform effective UAE. UAE is not contraindicated in a pedunculated subserosal leiomyoma. UAE in a cervical leiomyoma remains a challenging procedure. A leiomyoma with high signal intensity on T2-weighted imaging responds well to UAE, but a malignancy with similar radiological features should not be misdiagnosed as a leiomyoma. Administration of gonadotropin-releasing hormone agonists before UAE is useful in selected patients and is not a contraindication for the procedure. The risk of subsequent re-intervention 5 years after UAE is approximately 10%, which represents an acceptable profile. UAE for adenomyosis is challenging; initial embolization using small particles can achieve better success than that by using larger particles. An intravenous injection of dexamethasone prior to UAE, followed by a patient-controlled analgesia pump and intra-arterial administration of lidocaine after the procedure, are useful techniques to control pain. Dexmedetomidine is an excellent supplemental sedative, showing a fentanyl-sparing effect without causing respiratory depression. UAE for symptomatic leiomyoma is safe and can be an alternative to surgery in most patients with a low risk of re-intervention.
Uterine leiomyomas are the most common gynecological tumors, and approximately 50% of patients with these tumors experience symptoms (1). The American College of Obstetricians and Gynecologists has recommended uterine artery embolization (UAE) as a treatment option for selected women who wish to retain their uteri (2). Since the introduction of UAE for the treatment of symptomatic leiomyomas and adenomyosis, many studies have been published on the topic; some of the conclusions deserve further study, whereas other important outcomes do not shed much light on the topic. This review focuses on general knowledge regarding UAE but also highlights UAE-related concepts that are often not addressed, poorly understood, or rarely published.
This review discusses difficulties that are frequently encountered during UAE procedures and their solutions. Furthermore, recent trends in medication for pain control and updates regarding UAE for leiomyoma and adenomyosis are also presented.
According to the Society of Interventional Radiology guidelines, UAE is not contraindicated in patients with a pedunculated subserosal leiomyoma (3). In contrast, the Cardiovascular and Interventional Radiological Society of Europe guidelines consider a pedunculated subserosal leiomyoma as a relative contraindication due to the potential risks of detachment, infection, and sepsis (4). In a previous study of 1069 patients who underwent UAE, 55 patients with pedunculated subserosal leiomyomas, including 11 patients with high-risk pedunculated subserosal leiomyomas (stalk diameter < 25% of the leiomyoma diameter), showed no adverse events after UAE (5).
Most uterine leiomyomas (≥ 95%) develop in the uterine corpus, but a few (< 5%) occur in the uterine cervix. The location of a leiomyoma in the cervix increases the difficulty of surgery due to the limited access for suturing and increased blood loss (67). One study reported that the cervix always shows perfusion immediately after embolization, which may influence the results of UAE in cervical leiomyomas (8). Only 50% of cervical leiomyomas were successfully treated with UAE in a previous study (9). In the author's experience, the outcome depends on the vascularity of the leiomyoma during embolization (Fig. 1). Because cervical leiomyomas frequently show poor vascularity, the results of UAE were disappointing (9). Therefore, caution is required in the selection and pre-surgical counseling of patients, especially for those who are symptomatic only due to cervical leiomyomas.
High signal intensity (SI) of a leiomyoma on T2-weighted imaging (T2WI) may indicate high cellularity, proliferative activity, and increased vascularity (10). Cellular leiomyomas, which are composed of compact smooth muscle cells, have a relatively higher SI on T2WI and may demonstrate good enhancement on contrast-enhanced magnetic resonance (MR) imaging (MRI). Extensive edema in interstitial spaces may also result in high SI on T2WI. Burn et al. (11) reported that a high SI of leiomyomas on T2WI was a good prognostic factor. In a previous study (12), the mean volume reduction rate of leiomyomas in the T2-high group at three months after UAE was 61.7%, which was significantly higher than that in the control group (42.1%).
A few reports have discussed UAE for malignant tumors that were misdiagnosed as leiomyomas (1415). The US Food and Drug Administration has estimated that 1 in 350 women who undergo a hysterectomy or a myomectomy for presumptive leiomyoma have a uterine sarcoma, with an incidence between 0.24% and 1.4%. In our experience, among 1300 patients, two patients had a misdiagnosed malignancy, with an incidence of 0.15%.
The SI of a leiomyoma may vary on T2WI, making it difficult to differentiate a leiomyoma from a malignant tumor. Therefore, eliciting a detailed clinical history in addition to careful evaluation of the MR images is mandatory, even when a prior biopsy report is available (Fig. 3).
Controversy persists regarding the use of UAE for large leiomyomas (1617). Some authors insist that UAE for leiomyomas ≥ 10 cm in diameter should be avoided because of a higher incidence of complications such as infection, sepsis, uterine necrosis, and death, whereas others have reported no significant increase in complications for such large leiomyomas. One serious issue is the large leiomyoma becoming endocavitary after UAE, causing cramping abdominal pain with or without infection.
Gonadotropin-releasing hormone (GnRH) agonists are widely used preoperatively to reduce the size of leiomyomas. The common protocol involves subcutaneous administration of 3.75 mg of leuprolide acetate once a month for 2 to 6 months before UAE. Patients were monitored using ultrasound every month to determine the relative decrease in the size of leiomyomas. Because GnRH agonists tend to make the uterine arteries smaller and more spastic, radiologists might have some difficulty selecting the uterine arteries due to arterial spasm. However, although the uterine arteries in most patients who are treated with GnRH agonists shrink in comparison with their pre-treatment size, they are still sufficiently large to undergo UAE (18). Therefore, treatment with GnRH agonists does not preclude the use of UAE. Indeed, most of our patients treated with GnRH agonists demonstrated complete infarction of leiomyomas after the procedure (Fig. 4). Interestingly, patients who were treated with GnRH agonists experienced significantly less pain after the procedure compared with those who were not treated with GnRH agonists, according to the author's experience. Furthermore, fewer embolic materials were used in these patients.
HiFU is another option for non-surgical management of leiomyomas in selected patients. In contrast to UAE, which aims for complete infarction of leiomyomas in most patients, HiFU requires a peripheral, viable portion of the tumor to secure a safety margin. This could be the reason for the higher recurrence of leiomyomas following HiFU than that following UAE (Fig. 5). UAE can be considered a viable treatment option for recurrent or incompletely infarcted leiomyomas after HiFU.
UAE is usually not recommended for the treatment of leiomyomas in post-menopausal women. Some post-menopausal women with leiomyomas who take hormone replacement therapy develop symptoms, which can be persistent, and some experience vaginal bleeding. In a previous article, the growth of pre-existing leiomyomas or even development of new leiomyomas has been reported (19). However, UAE can be effective even in post-menopausal women (20) after the possibility of endometrial cancer is ruled out using MRI or endometrial biopsy.
Although deep vein thrombosis (DVT) followed by a pulmonary embolism after UAE is extremely rare, it can be fatal (Fig. 6). Nikolic et al. (21) revealed that UAE induced hypercoagulability. To date, four cases of mortality following UAE due to pulmonary embolism have been reported (2223). Because many patients with leiomyomas take oral contraceptives to control uterine bleeding, an unknown number of UAE candidates may already have DVT before the procedure. Hidden malignancy-related coagulopathy may also contribute to the development of DVT (Fig. 7). Therefore, recording a detailed clinical history and performing a routine d-dimer test before UAE can increase patient safety, specifically in those taking oral contraceptives.
Identification of leiomyomas using uterine arteriography is very important because it can predict the success of UAE (Fig. 8). In general, leiomyomas larger than 3 cm in diameter can be easily detected on arteriography by interventional radiologists with some experience. If a leiomyoma is not identified on an arteriogram, the possibility of a collateral supply should be considered, especially for large leiomyomas in the uterine fundus. Comparison of a coronal T2W MR image of the uterus with the fluoroscopic image during or after UAE is a good method to verify the accuracy of an embolization.
The uterine arteries may show some anatomical variations, such as a unilateral uterine artery supplying the entire uterus, duplicated uterine arteries, or an aplastic uterine artery replaced by an ovarian artery. Collaterals from the inferior mesenteric artery are identified at a significantly higher rate in patients with adenomyosis (24).
Highly tortuous uterine arteries can make it difficult for radiologists to select the arteries. In such cases, Robert's uterine catheter (Cook, Spencer, IN, USA) can be useful. In patients with a single uterine artery, the UAE outcome can be good if the leiomyoma receives its blood flow exclusively from the embolized uterine artery (Fig. 9).
A previous study demonstrated that 3.8% of patients who underwent UAE needed ovarian artery embolization (OAE) (25). OAE is required in patients with a Type II utero-ovarian anastomosis based on the utero-ovarian anastomosis classification (Fig. 10) (26), or with a hypoplastic or aplastic uterine artery functionally replaced by an ovarian artery (Fig. 11). Only patients with an ovarian artery entering the myometrium on aortography after UAE can be considered for OAE. MR angiography before UAE can be useful to predict the necessity of OAE (25). A Mickelson or Simmons Type II catheter (Cook) can be effective in selecting the ovarian artery.
Several investigations have described analgesic and pain management techniques to control pain after UAE. The most common method is a patient-controlled analgesia pump containing fentanyl and non-steroidal anti-inflammatory drugs. A recent randomized study revealed the efficacy of dexmedetomidine, which has a fentanyl-sparing effect and induces sedation without respiratory depression (27).
Administration of steroids may relieve pain and inflammation because steroids have anti-inflammatory effects. One previous study showed that a 10-mg intravenous injection of dexamethasone 1 hour before UAE induced a post-procedure decrease in the release of acute inflammation mediators C-reactive protein and interleukin-6, along with a decreased level of the stress hormone cortisol (28). Intra-arterial injection of 5 mL of 1% lidocaine administered after UAE into each uterine artery is also effective for acute pain relief (29).
Endocavitary leiomyoma may occur following UAE (33), inducing serious complications such as secondary infection. Patients with endocavitary leiomyoma with or without infection may present with symptoms of cramping abdominal pain, vaginal discharge with a foul odor, and fever. In severe cases, hysteroscopic myomectomy with cervical dilation and curettage or, rarely, hysterectomy may be needed. When a necrotic leiomyoma cannot be completely excised the first time, cervical dilation and curettage or hysteroscopic resection can be attempted more than 2 or 3 times for complete removal (Fig. 12). However, endocavitary leiomyomas less than 6 cm in diameter are not generally considered clinically significant according to the author's experience.
In the author's limited experience, the outcome of repeated UAE was disappointing. On arteriography, some patients have uterine arteries that have recanalized or have several collaterals from pelvic branches or ovarian arteries, rendering repeat UAE inefficient (Fig. 13). Therefore, repeated UAE is not usually recommended.
Previous randomized studies that compared the long-term effectiveness of UAE and surgery for leiomyomas revealed that the re-intervention rate at 5 years was significantly higher for UAE (32%) than for surgery (4%) (3435). However, these data included procedures performed during the early periods of UAE, when the technical success rates were low. The rate of complete necrosis of leiomyomas with UAE during early attempts was also low. However, previous studies did not address an essential query: what percentage of patients would need re-intervention in 5 years after UAE when complete necrosis of leiomyomas had been achieved as observed using MRI after UAE?
One recent study found that only approximately 10% of patients who underwent UAE, most of which showed complete necrosis of leiomyomas confirmed using MRI, required a re-intervention procedure such as myomectomy or hysterectomy at 5 years (36). We believe that this rate might be acceptable, indicating the sustainability of UAE.
Adenomyosis is characterized by the presence of endometrial glands and stroma scattered randomly deep within the myometrium, along with adjacent smooth muscle hyperplasia. Approximately one-third of women with adenomyosis experience symptoms such as menorrhagia, pelvic pain, and bulk-related symptoms. Adenomyosis is regarded as a difficult disease entity to treat, with hysterectomy considered the only curative treatment. The long-term efficacy of UAE as a treatment option for adenomyosis remains controversial and is lower than its efficacy for treating leiomyoma. Previous studies (37383940) have revealed that when small non-spherical polyvinyl alcohol (PVA) particles measuring 150–250 µm are used at the beginning of embolization, followed by 250–355-µm and 355–500-µm particles (the “1-2-3 protocol”), complete necrosis of adenomyosis was achieved in more than 80% of patients after UAE, with a low recurrence rate of symptoms (Fig. 14). Angiography usually shows a straight, dilated, and perifibroid plexus in leiomyomas, whereas only fine myometrial staining is seen in adenomyosis. Therefore, using medium-sized embolic particles (355–500 µm PVA, or 500–700 µm Tris-acryl gelatin microspheres) for UAE in adenomyosis might be less effective in achieving ischemia of the uterus. However, there could be concerns regarding the use of small PVA particles. In contrast to our expectation, however, the use of small 150- to 250-µm-sized particles during UAE was reported to be safe and effective for leiomyomas (41). In this study, only one patient experienced permanent amenorrhea due to endometrial atrophy with normal hormonal levels. Therefore, the use of small particles may be safe and effective. However, because endometrial atrophy causes infertility, small particles should not be recommended for women who desire a future pregnancy. A dark SI of adenomyosis on T2WI (similar to the SI of rectus muscle) or continuous junctional zone thickening from the endometrium with a homogeneously low SI were findings that predicted a favorable response to UAE. Conversely, a heterogeneous SI or an SI equal to that of the myometrium was an unfavorable predictor (37).
UAE can be an effective alternative treatment to surgery in most patients with symptomatic leiomyomas. Understanding the anatomy of a normal uterine artery and its variations is crucial. If patients are appropriately screened prior to UAE and the outcome is expected to be successful based on post-procedural imaging, the risk of re-intervention is low. Proper management of post-procedural pain and other complications is essential. GnRH agonists can be used as pre-UAE treatment for patients with large leiomyomas. In adenomyosis, achievement of complete necrosis with UAE is still challenging, but the use of small embolic particles can be safe and effective.
References
1. Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015; 372:1646–1655. PMID: 25901428.
2. American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008; 112(2 Pt 1):387–400. PMID: 18669742.
3. Dariushnia SR, Nikolic B, Stokes LS, Spies JB. Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for uterine artery embolization for symptomatic leiomyomata. J Vasc Interv Radiol. 2014; 25:1737–1747. PMID: 25442136.

4. van Overhagen H, Reekers JA. Uterine artery embolization for symptomatic leiomyomata. Cardiovasc Intervent Radiol. 2015; 38:536–542. PMID: 25465064.

5. Kim YS, Han K, Kim MD, Kim GM, Kwon JH, Lee J, et al. Uterine artery embolization for pedunculated subserosal leiomyomas: evidence of safety and efficacy. J Vasc Interv Radiol. 2018; 29:497–501. PMID: 29477623.

6. Sinha R, Sundaram M, Lakhotia S, Hegde A. Cervical myomectomy with uterine artery ligation at its origin. J Minim Invasive Gynecol. 2009; 16:604–608. PMID: 19835802.

7. Takeuchi H, Kitade M, Kikuchi I, Shimanuki H, Kumakiri J, Kobayashi Y, et al. A new enucleation method for cervical myoma via laparoscopy. J Minim Invasive Gynecol. 2006; 13:334–336. PMID: 16825077.

8. Scheurig-Muenkler C, Wagner M, Franiel T, Hamm B, Kroencke TJ. Effect of uterine artery embolization on uterine and leiomyoma perfusion: evidence of transient myometrial ischemia on magnetic resonance imaging. J Vasc Interv Radiol. 2010; 21:1347–1353. PMID: 20685136.

9. Kim MD, Lee M, Jung DC, Park SI, Lee MS, Won JY, et al. Limited efficacy of uterine artery embolization for cervical leiomyomas. J Vasc Interv Radiol. 2012; 23:236–240. PMID: 22177843.

10. Murase E, Siegelman ES, Outwater EK, Perez-Jaffe LA, Tureck RW. Uterine leiomyomas: histopathologic features, MR imaging findings, differential diagnosis, and treatment. Radiographics. 1999; 19:1179–1197. PMID: 10489175.

11. Burn PR, McCall JM, Chinn RJ, Vashisht A, Smith JR, Healy JC. Uterine fibroleiomyoma: MR imaging appearances before and after embolization of uterine arteries. Radiology. 2000; 214:729–734. PMID: 10715038.

12. Chang S, Kim MD, Lee M, Lee MS, Park SI, Won JY, et al. Uterine artery embolization for symptomatic fibroids with high signal intensity on T2-weighted MR imaging. Korean J Radiol. 2012; 13:618–624. PMID: 22977330.

13. Funaki K, Fukunishi H, Funaki T, Kawakami C. Mid-term outcome of magnetic resonance-guided focused ultrasound surgery for uterine myomas: from six to twelve months after volume reduction. J Minim Invasive Gynecol. 2007; 14:616–621. PMID: 17848324.

14. Coffin P, Ascher S, Spies J. Risk of uterine malignancy in a population presenting for uterine artery embolization. J Vasc Interv Radiol. 2016; 27:S18.

15. Kainsbak J, Hansen ES, Dueholm M. Literature review of outcomes and prevalence and case report of leiomyosarcomas and non-typical uterine smooth muscle leiomyoma tumors treated with uterine artery embolization. Eur J Obstet Gynecol Reprod Biol. 2015; 191:130–137. PMID: 26117442.

16. Katsumori T, Nakajima K, Mihara T. Is a large fibroid a high-risk factor for uterine artery embolization? AJR Am J Roentgenol. 2003; 181:1309–1314. PMID: 14573425.

17. Worthington-Kirsch RL, Andrews RT, Siskin GP, Shlansky-Goldberg R, Lipman JC, Goodwin SC, et al. II. Uterine fibroid embolization: technical aspects. Tech Vasc Interv Radiol. 2002; 5:17–34. PMID: 12098105.

18. Kim MD, Lee M, Lee MS, Park SI, Wonq JY, Lee DY, et al. Uterine artery embolization of large fibroids: comparative study of procedure with and without pretreatment gonadotropin-releasing hormone agonists. AJR Am J Roentgenol. 2012; 199:441–446. PMID: 22826410.

19. Yang CH, Lee JN, Hsu SC, Kuo CH, Tsai EM. Effect of hormone replacement therapy on uterine fibroids in postmenopausal women--a 3-year study. Maturitas. 2002; 43:35–39. PMID: 12270580.

20. Lee SJ, Kim MD, Kim GM, Won JY, Park SI, Lee DY. Uterine artery embolization for symptomatic fibroids in postmenopausal women. Clin Imaging. 2016; 40:106–109. PMID: 26372352.

21. Nikolic B, Kessler CM, Jacobs HM, Abbara S, Ammann AM, Neeman Z, et al. Changes in blood coagulation markers associated with uterine artery embolization for leiomyomata. J Vasc Interv Radiol. 2003; 14(9 Pt 1):1147–1153. PMID: 14514806.

22. Hamoda H, Tait P, Edmonds DK. Fatal pulmonary embolus after uterine artery fibroid embolisation. Cardiovasc Intervent Radiol. 2009; 32:1080–1082. PMID: 19449063.

23. Lanocita R. A fatal complication of percutaneous transcatheter embolization for treatment of uterine fibroids. In : Second international symposium on embolization of uterine myomata; 1999 September 16–18; Boston, MA, USA.
24. Chang S, Lee MS, Kim MD, Yoon CJ, Jung DC, Lee M, et al. Inferior mesenteric artery collaterals to the uterus during uterine artery embolization: prevalence, risk factors, and clinical outcomes. J Vasc Interv Radiol. 2013; 24:1353–1360. PMID: 23891048.

25. Lee MS, Kim MD, Lee M, Won JY, Park SI, Lee DY, et al. Contrast-enhanced MR angiography of uterine arteries for the prediction of ovarian artery embolization in 349 patients. J Vasc Interv Radiol. 2012; 23:1174–1179. PMID: 22920980.

26. Razavi MK, Wolanske KA, Hwang GL, Sze DY, Kee ST, Dake MD. Angiographic classification of ovarian artery-to-uterine artery anastomoses: initial observations in uterine fibroid embolization. Radiology. 2002; 224:707–712. PMID: 12202703.

27. Kim SY, Chang CH, Lee JS, Kim YJ, Kim MD, Han DW. Comparison of the efficacy of dexmedetomidine plus fentanyl patient-controlled analgesia with fentanyl patient-controlled analgesia for pain control in uterine artery embolization for symptomatic fibroid tumors or adenomyosis: a prospective, randomized study. J Vasc Interv Radiol. 2013; 24:779–786. PMID: 23707085.

28. Kim SY, Koo BN, Shin CS, Ban M, Han K, Kim MD. The effects of single-dose dexamethasone on inflammatory response and pain after uterine artery embolisation for symptomatic fibroids or adenomyosis: a randomised controlled study. BJOG. 2016; 123:580–587. PMID: 26667403.

29. Noel-Lamy M, Tan KT, Simons ME, Sniderman KW, Mironov O, Rajan DK. Intraarterial lidocaine for pain control in uterine artery embolization: a prospective, randomized study. J Vasc Interv Radiol. 2017; 28:16–22. PMID: 27884686.

30. Yoon J, Valenti D, Muchantef K, Cabrera T, Toonsi F, Torres C, et al. Superior hypogastric nerve block as post-uterine artery embolization analgesia: a randomized and double-blind clinical trial. Radiology. 2018; 289:248–254. PMID: 29989515.

31. Rasuli P, Jolly EE, Hammond I, French GJ, Preston R, Goulet S, et al. Superior hypogastric nerve block for pain control in outpatient uterine artery embolization. J Vasc Interv Radiol. 2004; 15:1423–1429. PMID: 15590800.

32. Pereira K, Salamo RM, Morel-Ovalle LM, Patel N, Patel R. Ropivacaine-induced local anesthetic systemic toxicity after superior hypogastric nerve block for pain control after uterine artery embolization. J Vasc Interv Radiol. 2018; 29:1315–1317. PMID: 30146211.

33. Park HR, Kim MD, Kim NK, Kim HJ, Yoon SW, Park WK, et al. Uterine restoration after repeated sloughing of fibroids or vaginal expulsion following uterine artery embolization. Eur Radiol. 2005; 15:1850–1854. PMID: 15729564.

34. van der Kooij SM, Hehenkamp WJ, Volkers NA, Birnie E, Ankum WM, Reekers JA. Uterine artery embolization vs hysterectomy in the treatment of symptomatic uterine fibroids: 5-year outcome from the randomized EMMY trial. Am J Obstet Gynecol. 2010; 203:105.e1–105.e13. PMID: 20579960.

35. Moss JG, Cooper KG, Khaund A, Murray LS, Murray GD, Wu O, et al. Randomised comparison of uterine artery embolisation (UAE) with surgical treatment in patients with symptomatic uterine fibroids (REST trial): 5-year results. BJOG. 2011; 118:936–944. PMID: 21481151.

36. Yoon JK, Han K, Kim MD, Kim GM, Kwon JH, Won JY, et al. Five-year clinical outcomes of uterine artery embolization for symptomatic leiomyomas: an analysis of risk factors for reintervention. Eur J Radiol. 2018; 109:83–87. PMID: 30527317.

37. Kim MD, Kim YM, Kim HC, Cho JH, Kang HG, Lee C, et al. Uterine artery embolization for symptomatic adenomyosis: a new technical development of the 1-2-3 protocol and predictive factors of MR imaging affecting outcomes. J Vasc Interv Radiol. 2011; 22:497–502. PMID: 21377897.

38. Jung DC, Kim MD, Oh YT, Won JY, Lee DY. Prediction of early response to uterine arterial embolisation of adenomyosis: value of T2 signal intensity ratio of adenomyosis. Eur Radiol. 2012; 22:2044–2049. PMID: 22527376.

39. Bae SH, Kim MD, Kim GM, Lee SJ, Park SI, Won JY, et al. Uterine artery embolization for adenomyosis: percentage of necrosis predicts midterm clinical recurrence. J Vasc Interv Radiol. 2015; 26:1290–1296.e2. PMID: 26074028.

40. Park Y, Kim MD, Jung DC, Lee SJ, Kim G, Park SI, et al. Can measurement of apparent diffusion coefficient before treatment predict the response to uterine artery embolization for adenomyosis? Eur Radiol. 2015; 25:1303–1309. PMID: 25481638.

41. Tropeano G, Litwicka K, Di Stasi C, Romano D, Mancuso S. Permanent amenorrhea associated with endometrial atrophy after uterine artery embolization for symptomatic uterine fibroids. Fertil Steril. 2003; 79:132–135. PMID: 12524076.

Fig. 1
44-year-old woman presenting with heavy bleeding due to cervical leiomyoma.
A. Sagittal T2WI MRI shows 7-cm cervical leiomyoma (arrows). B. Uterine arteriography reveals hypovascular leiomyoma (arrows). C. One month after UAE, patient presented with vaginal expulsion of leiomyoma, which was partially infarcted and viable (arrows) on contrast-enhanced MRI. D. Hysteroscopic resection was performed under general anesthesia. T2WI = T2-weighted imaging, UAE = uterine artery embolization
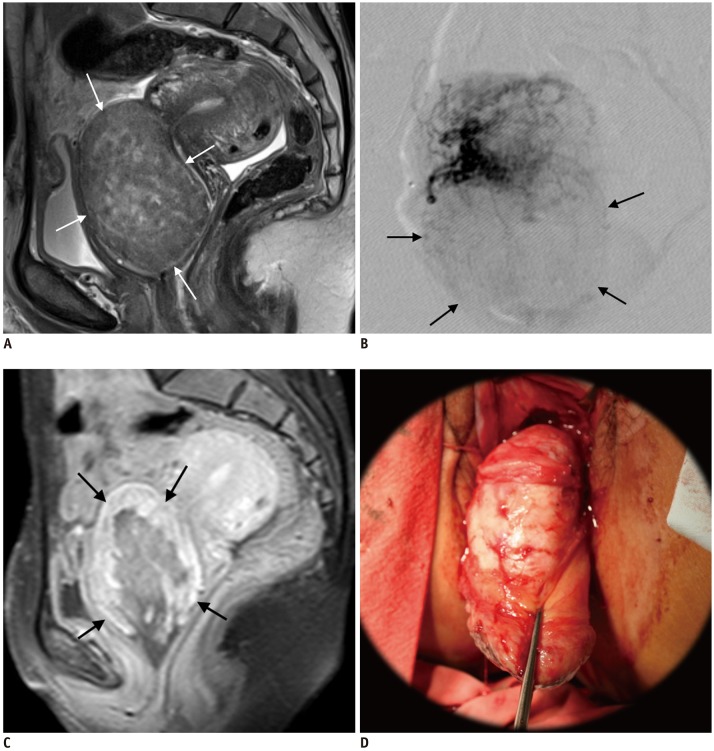
Fig. 2
Leiomyoma with high SI on T2WI.
A. 41-year-old woman with leiomyoma with high SI on T2WI (arrows). B. Patient underwent failed HiFU. UAE was performed, and 3-month follow-up MRI showed complete necrosis of leiomyoma (asterisk). HiFU = high-intensity focused ultrasound, SI = signal intensity
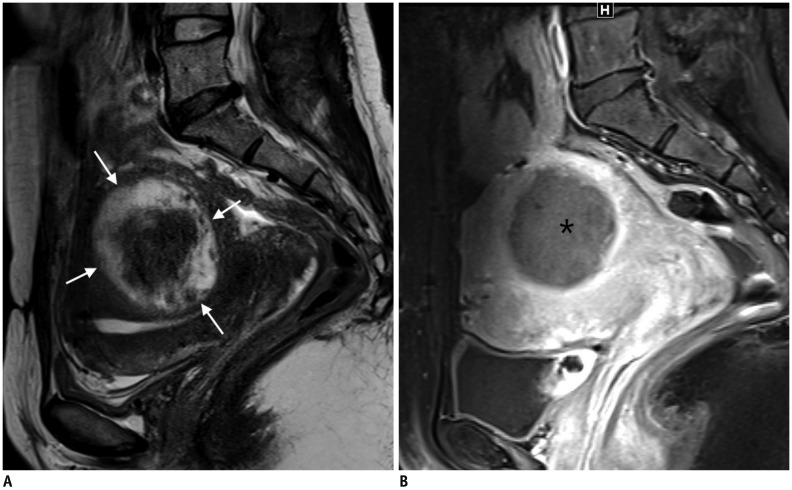
Fig. 3
UAE for malignant tumors misdiagnosed as leiomyomas.
31-year-old woman underwent myomectomy at another hospital 4 months prior, and histopathological examination revealed leiomyoma.A. MRI showed tumor 7 cm in size (arrows) with high SI; thus, leiomyoma was diagnosed based on pathologic report. B. 3-month follow-up MRI after UAE revealed exacerbation of uterine tumor with peritoneal seeding (arrows) as well as seeding along incision scar. Spindle cell sarcoma of uterus was confirmed.
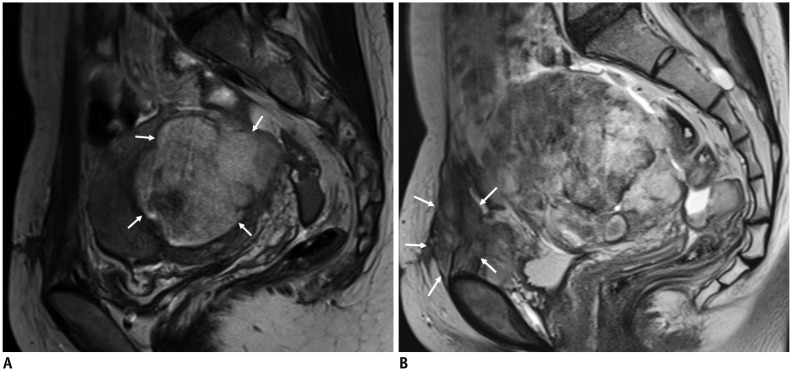
Fig. 4
GnRH agonists for large leiomyoma prior to UAE.
A. 46-year-old woman presented with voiding difficulty. MRI revealed 10-cm large leiomyoma (arrows). B. After two doses of GnRH agonists, leiomyoma reduced to 4.7 cm in size (arrowheads), 85% volume reductio. C. Left uterine arteriography demonstrated leiomyoma (arrows). D. Complete infarction of leiomyoma (asterisk) was seen on 3-month follow-up MRI. GnRH = gonadotropin-releasing hormone
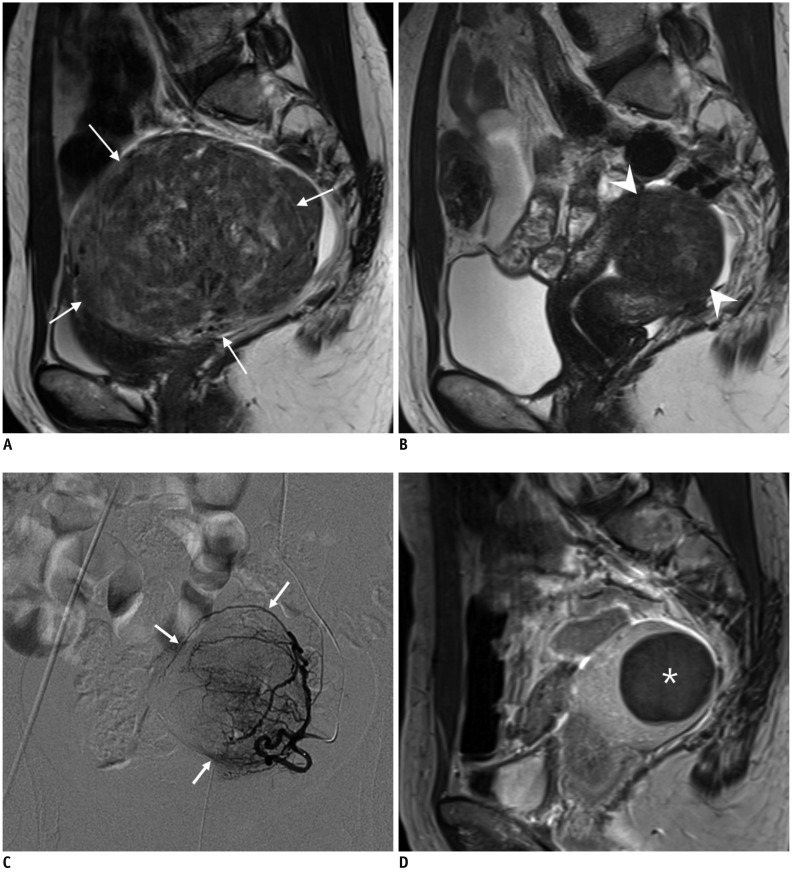
Fig. 5
44-year-old woman presenting with recurrent leiomyoma after HiFU.
Serial MRI revealed complete regrowth of leiomyoma (arrows) 26 months after HiFU. Patient underwent UAE, and 3-month follow-up MRI revealed complete infarction of leiomyoma (arrowheads). mo = month
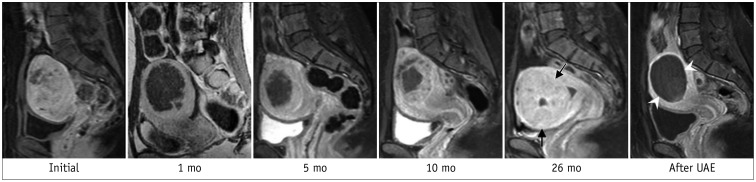
Fig. 6
Pulmonary embolism after UAE for adenomyosis.
40-year-old woman with adenomyosis who was taking oral contraceptive pills for 3 weeks underwent UAE. Ten days after UAE, patient experienced dyspnea, and CT (A) revealed multiple pulmonary emboli (arrows) and thrombosis (arrow) of right common femoral vein (B). (Courtesy of Dr. Yeon Jaewoo at Bundang Jaeseng General Hospital).
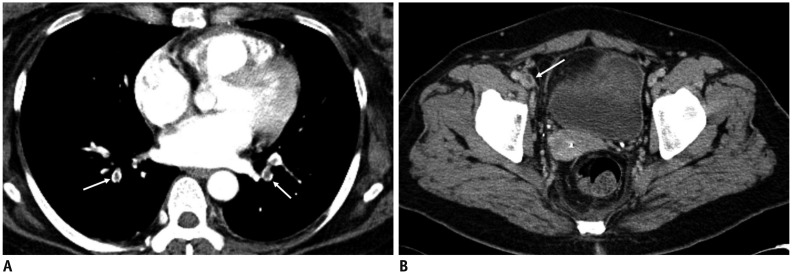
Fig. 7
Identification of DVT before UAE.
50-year-old woman with adenomyosis and leiomyoma presented with uterine bleeding. She had been treated with oral contraceptives for 2 weeks. D-dimer level was 1143 ng/mL (normal range: 0–243 ng/mL), and CT scan of her lower extremities revealed venous thrombosis (arrow) in left lower leg. Renal cell carcinoma was detected incidentally (not shown). Partial nephrectomy was performed. One-month follow-up CT scan demonstrated complete occlusion of inferior vena cava filter and progression of thrombosis along both common iliac veins (not shown). DVT = deep vein thrombosis
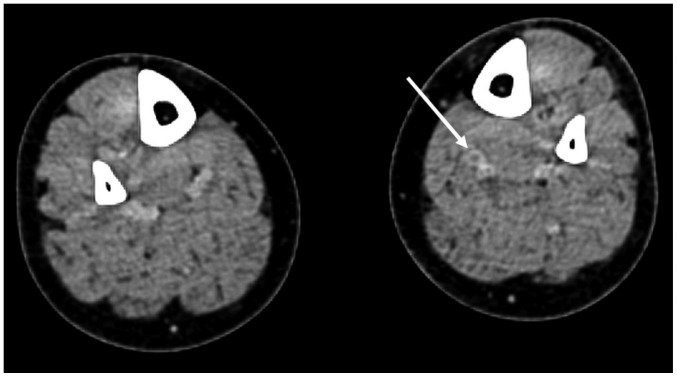
Fig. 8
Identification of leiomyoma in angiography.
Uterine arteriography reveals leiomyoma (arrows). Right ovary (asterisk) is seen, representing example of Type III utero-ovarian anastomosis based on Razavi's classification.
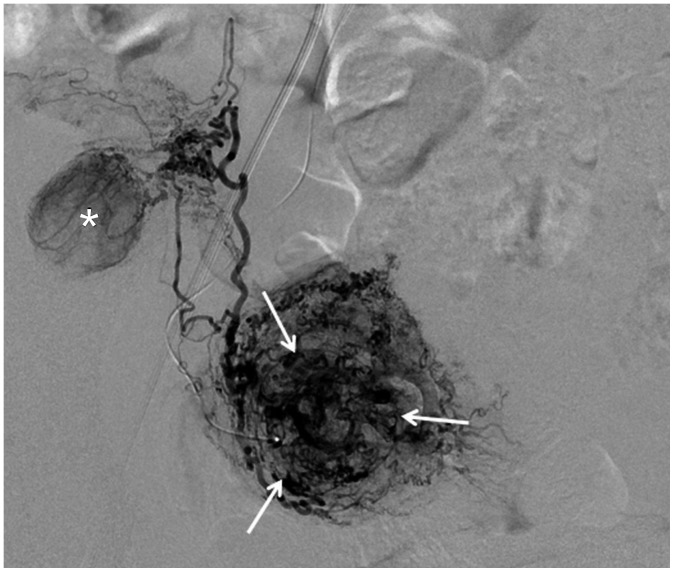
Fig. 9
Leiomyoma receiving blood flow from unilateral uterine artery.
43-year-old woman underwent kidney transplantation 15 years prior. A. Abdominal aortography showed total occlusion of right internal iliac artery with reconstitution of right uterine artery (arrow) via collaterals. B. On selective uterine arteriography on left side, leiomyoma (arrows) received blood flow exclusively from left uterine artery. C. Complete devascularization of leiomyoma (arrows) is noted at 3-month follow-up contrast-enhanced ultrasound scan. Contrast-enhanced ultrasound instead of MRI was used due to end-stage renal disease.
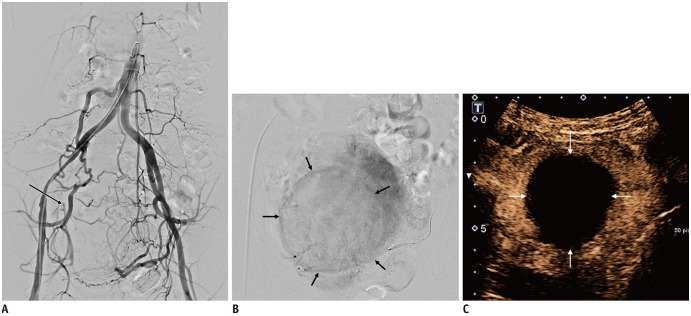
Fig. 10
Ovarian artery collateral to leiomyoma in uterine fundus.
44-year-old woman presented with multiple leiomyomas. A. MR angiography revealed hypertrophy of both uterine and ovarian (arrows) arteries. B. Leiomyoma on uterine fundus was supplied by left ovarian artery (Type II anastomosis). MR = magnetic resonance
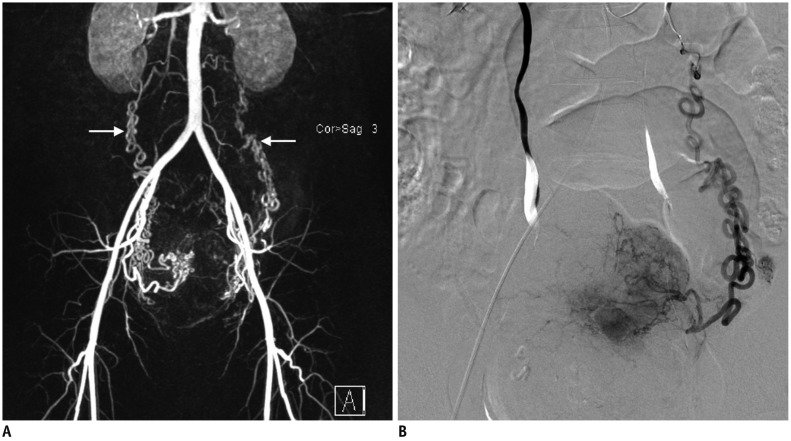
Fig. 11
Aplasia of right uterine artery.
MR angiography revealed presence of only left uterine artery (arrow). Patient required right ovarian artery embolization.
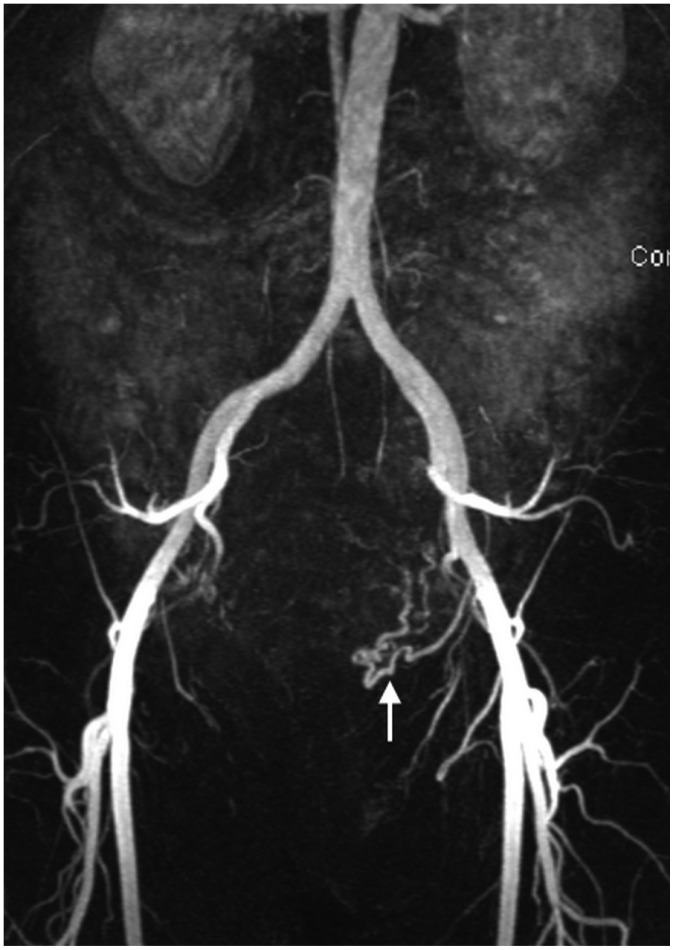
Fig. 12
Leiomyoma becoming endocavitary after UAE.
52-year-old woman had dysfunctional uterine bleeding. A. Sagittal T2WI revealed 7-cm pedunculated submucosal leiomyoma (arrows). B. One month after UAE, patient presented with cramping abdominal pain. Gadolinium-enhanced MRI revealed complete necrosis of submucosal leiomyoma (asterisk), which had become endocavitary, along with widening of cervix, suggesting ongoing expulsion. Three cervical dilatation and curettage procedures were needed to completely remove necrotic leiomyoma.
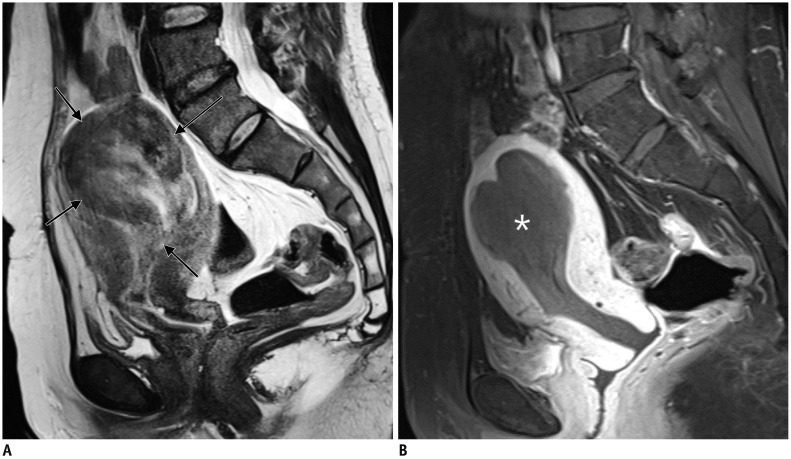
Fig. 13
Repeated UAE.
43-year-old woman who had undergone UAE for leiomyomas 8 years prior presented with evidence of recurrent leiomyomas.A. Aortography revealed total occlusion of right uterine artery and hypertrophied right ovarian artery (arrows). B. Left uterine artery was partially recanalized, but multiple fine collaterals (arrows) supplied leiomyoma and uterus. Follow-up MRI performed after repeated UAE showed that procedure was ineffective (not shown).
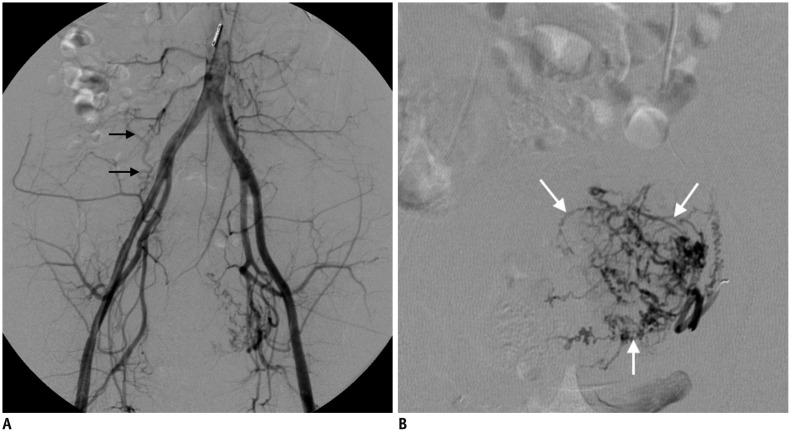
Fig. 14
38-year-old woman with adenomyosis presenting with vaginal bleeding and pain during menstruation.
A. MRI revealed focal adenomyosis (arrows) with homogeneous, continuous, junctional zone thickening from endometrium. B. After embolization using small polyvinyl alcohol particles (1-2-3 protocol), complete necrosis of adenomyosis (arrows) was seen on 3-month follow-up MRI. Patient's status has been stable for 5 years.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download