Abstract
Current knowledge of functional tricuspid regurgitation (FTR) as a progressive entity, worsening the prognosis of patients irrespective of its aetiology, has led to renewed interest in the pathophysiology and assessment of FTR. For the proper management of FTR, not only its severity, but also the mechanisms, the mode of leaflet coaptation, the degree of tricuspid annulus enlargement and leaflet tenting, and the haemodynamic consequences for right atrial and right ventricular morphology and function have to be taken into account. A better assessment of the anatomy and function of tricuspid apparatus and tricuspid regurgitation severity should help with the appropriate selection of patients who will benefit from either surgical tricuspid valve repair/replacement or a percutaneous procedure, especially among patients who are to undergo or have undergone primary left-sided valvular surgery. In this article, we review the anatomy, pathophysiology and the use of imaging techniques to assess patients with FTR, as well as the various treatment options for FTR, including emerging transcatheter procedures. The limitations affecting the current approach to FTR patients and the unmet clinical needs for their management have also been discussed.
Functional or secondary tricuspid regurgitation (FTR) typically refers to tricuspid regurgitation occurring as a consequence of geometric deformation of the tricuspid apparatus, in the absence of structural lesions of a tricuspid valve (TV). Common conditions evolving with functional tricuspid regurgitation (TR) are left-sided heart valve diseases, left ventricular (LV) dysfunction, congenital heart defects (atrial septal defects, corrected tetralogy of Fallot etc.), pulmonary hypertension, right ventricular (RV) cardiomyopathy and RV infarction.
FTR is not a benign disease, as previously thought.1)2) If left untreated, FTR progresses over the years and has been witnessed in a sizable number of patients;3) severe TR is associated with poorer survival, increased morbidity and reduced functional capacity.4)5) At present, referral for surgical correction of FTR is often delayed until intractable right heart failure develops, when patient outcome is poor and operative mortality becomes significant.6)7) Conversely, there is good evidence that TV annuloplasty is a low risk procedure and concomitant TV repair does not significantly increase the perioperative mortality and morbidity when correcting left sided valve diseases.8) Appropriate patient selection and timing for TV repair or replacement are crucial for optimal outcome, but objective criteria for clinical decison-making remain poorly defined.9)
This review presents the anatomy of TV in relation with the pathophysiology of FTR, the use of imaging techniques to assess its severity, as well as the current management of FTR patients, including surgical and percutaneous strategies for TV repair. The limitations affecting the current approach to FTR patients and the unmet clinical needs for their management will also be discussed.
The TV is the largest and most caudally located among the four cardiac valves. The tricuspid valve is more variable and complex in morphology than the mitral valve. Compared to the mitral valve which has two leaflets, the TV classically consists of three leaflets assigned anatomically as the septal, anterior and posterior (also named mural or inferior). However, in some patients, the word "tricuspid" may be a misnomer, as autopsy studies have reported a great variability in the number of leaflets of TV (from to 2 to 6).10)11) Normal TV leaflets are thinner than the mitral valve leaflets due to lower right-sided pressures. TV leaflets are of unequal size: anterior leaflet is the largest, septal leaflet is the smallest, while posterior leaflet has multiple scallops and is of intermediate size. The septal leaflet arises from part of the annulus that is relatively fixed, due to its position between the fibrous trigone, and its insertion is characteristically apical relative to the septal insertion of the anterior mitral leaflet. As opposed to the mitral valve, TV shows a unique septal attachment (multiple attachments from the septal leaflet to the ventricular septum), and the lack of fibrous continuity with the semilunar valve (i.e. pulmonary valve) due to the interposition of crista supraventricularis.12)
Effective TV function depends on the structural integrity and functional coordination of all components of TV apparatus, which include leaflets, annulus (TA), chordae tendineae, papillary muscles and ventricular myocardium. Normal TA has an elliptical nonplanar shape, with the postero-septal portion being lowest (towards the RV apex) and the antero-septal portion the highest. Its shape is more flattened and oval than the saddle-shaped mitral annulus, and TA diameters, circumference and area are all larger than those of mitral annulus by approximately 20%. The maximal normal TA diameter in 4-chamber view in diastole is 19±2 mm/m2. Many factors influence normal TA size, including age, gender, right atrial (RA) and RV size.13) In addition, TA can change markedly with loading conditions and is a highly dynamic structure during cardiac cycle (with 25% fractional shortening and 30-40% of area decrease during atrial systole),14) holding important implications for its sizing.
The diastolic opening of the TV along with corresponding expansion of the annulus provides a tricuspid orifice area of 7-9 cm2.
The papillary muscles are also different than those supporting the mitral valve: smaller, often multiple, widely separated, and carrying chordae to the homolateral TV leaflet(s). Moreover, there may be accessory chordal attachments to the RV free wall and to the moderator band. These complex and multiple chordal connections play an important role in the genesis of FTR, as RV enlargement and dysfunction directly affect proper leaflet coaptation. Whereas the separation of mitral leaflets by annular enlargement is limited by the crossing of chordae tendineae (as each papillary muscle provides chordae to both mitral leaflets), the links of tricuspid leaflets only to homolateral papillary muscles allows wide separation of TV leaflets with annular and cavity enlargement.
Annular dilation is often present in patients with significant TR, and the importance of the TA in maintaining normal TV function cannot be overemphasized. Since the septal leaflet is fixed and the posterior leaflet is attached to the inferior wall of the RV (whose displacement is limited by the diaphragm), the TA can only dilate along the anterior leaflet attachments as the RV free wall expands outward. As the TA dilates, its morphology alters, becoming more planar and more circular.14)15) TR occurs when TA dilation is sufficient enough to reduce the TV leaflet coaptation height at a minimum.16)17)
Functional TR results from geometrical distortion of the normal spatial relationships of the TV leaflets, annulus, chords, papillary muscles, and RV walls.
The pathophysiology of FTR involves the following mechanisms: i. Dilation of the TA secondary to RV dilation predominantly along the RV free wall and/or RA dilation; ii. Changes in the 3 dimension-geometry and dynamics of the TA (especially with significant TR), i.e. TA becomes larger, flatter, more circular, with impaired areas shortening and excursions ocurring during the cardiac cycle; iii. With worsening FTR, subsequent RV and RA dilation lead to a distortion of the normal geometrical relationships of the TV leaflets, chordaes, and papillary muscles, resulting in tethering of the tricuspid leaflets and in incomplete closure with loss of coaptation zone (Fig. 1).16)
It is also important to note that atrial fibrillation, especially if chronic, can contribute to the development of FTR, mainly through its effect on RA size with subsequent TA dilation.18) In addition, the volume load of the TR itself can lead to FTR worsening due to increased RV and RA dilation, further exacerbating annular dilation and FTR (Fig. 1).
The role of TA in FTR development emerged since the 1970s, when it was noted that an enlarged TA is a constant feature in patients with FTR. The size of TA is correlated with the severity of FTR, and is a marker of a "diseased TV", predicting the propensity for future regurgitation irrespective of the actual degree of current FTR. For that reason, and since FTR severity assessment has many limitations, TA dilation is increasingly used as a more reliable indication for concurrent TV repair than FTR severity.9)
In clinical practice, echocardiographic assessment of TR includes integration of data from 2 dimension echocardiography (DE) and 3DE imaging of TV, right heart chambers size and function, interventricular septal motion and inferior vena cava (IVC), as well as Doppler parameters of regurgitation severity 2DE combined with spectral and color flow Doppler is the main modality used for detection and quantitation of TR.19) "Physiological" FTR is a frequent finding in healthy subjects and is typically associated with normal TV leaflet morphology, normal RV and RA size.20) The color jet is localized in a small region adjacent to the valve closure, is thin, central and often limited to early-systole.
Comprehensive evaluation of leaflet morphology and of the pathophysiological mechanisms underlying TR is warranted whenever an abnormal or "pathologic" FTR of more than mild severity is encountered. In such cases, the use of transthoracic 3DE may provide important clues regarding aetiology and mechanisms of valve dysfunction.21)22) 3DE has the unique capability to provide en face visualizations of the functional anatomy of TV leaflets and TA from the atrial side ("surgical view") in the beating heart (Fig. 2). When transthoracic 3DE is unsatisfactory, transoesophageal 3DE may provide a more detailed assessment of TV morphology and function, even though the visualization of TV may be more challenging than for left-sided valves.
Functional TR is characterized by annular dilatation (>40 mm or 21 mm/m2 in apical 4-chamber view) and/or by leaflet tethering (tenting distance>8 mm, tenting area>1.6 cm2).23) In most severe cases, the leaflets have lost the normal coaptation point, resulting in wide-open regurgitation.
Color flow Doppler and spectral Doppler are generally used for the semi-quantitative assessment of FTR severity.19) In clinical practice, the echocardiographer first performs a visual estimate of TR severity based on color Doppler jet characteristics, using multiple windows for FTR sampling: parasternal (tricuspid inflow view and short-axis view at great vessels level), apical (4-chamber and RV-focused view) or subcostal (4-chamber view). Small thin central jets usually indicate a mild FTR. Conversely, if an eccentric jet is found, the regurgitation is most likely significant and organic rather than functional. Evaluation of FTR by color Doppler jet area, despite its simplicity, is limited by technical and haemodynamic factors and therefore it is no longer recommended to assess TR severity when it is more than mild.19) A more quantitative FTR assessment is provided by vena contracta (VC) width and proximal isovelocity surface area (PISA) measurements.
VC represents the cross-sectional area of the blood column as it leaves the regurgitant orifice, and thereby reflects the regurgitant orifice area. The VC of the TR flow is typically imaged in the apical 4-chamber view using a careful probe angulation to optimize the flow image, an adapted Nyquist limit (color Doppler scale, 40-70 cm/s) to identify with clarity the neck of the jet and a narrow sector scan coupled with the zoom mode to maximize temporal resolution and measurement accuracy.24) Averaging measurements over three consecutive beats is recommended. Vena contracta width>6.5 mm (7.0 mm in the recent American College of Cardiology/American Heart Association (ACC/AHA) guidelines25) is usually associated to severe TR. Intermediate values are not accurate for distinguishing moderate from mild TR. A limitation of measuring VC width is the fact that regurgitant orifice geometry in case of FTR is generally either elliptical or complex star-shaped, and only rarely circular (Fig. 3). Moreover, its longer diameter is oriented in the antero-posterior direction,26) therefore it does not coincide with the VC width displayed in apical 4-chamber view, which frequently underestimates the FTR severity (Fig. 3). These limitations may explain the moderate correlation between VC width by 2D color Doppler and 3DE planimetry of vena contracta area (Fig. 4).27) The reported cutoffs of vena contracta area by color 3DE suggestive of severe TR were >0.57 cm2 in FTR and >0.36 cm2 regardless of TR mechanism.26)27) Influence of technical factors (inadequate breath holding, gain changes, color baseline adjustments, low temporal resolution and spatial resolution by transthoracic approach) and arrhythmias are limiting the clinical implementation of this method.
PISA radius measurement is by itself a good indicator of severity of regurgitation, but complete application of the method allows to obtain quantitative measures of FTR, such as effective regurgitant orifice area (EROA) and regurgitant volume. For an optimal visualization of the PISA, the apical 4-chamber view focused on the RV and the parasternal views are generally recommended. The area of interest is optimized by zooming the image and setting the Nyquist limit to approximately 10-20% of the peak velocity of the TR jet. The PISA radius is measured at mid-systole using the first aliasing. A PISA radius>9 mm at a Nyquist limit of 28 cm/s has been associated with the presence of significant TR (corresponding to an effective regurgitant orifice area≥40 mm2 and a regurgitant volume≥45 mL, the quantitative thresholds for severe TR), whereas a radius less than 5 mm suggests mild TR. However, the PISA method may underestimate the severity of TR by 30% and faces several limitations,28) particularly in eccentric jets and in severely tethered leaflets (in which the outer angle formed by these should be accounted for in the calculation of regurgitant flow). Many patients with FTR show important respiratory variations of TR velocity and PISA radius, in which case average measurements during inspiration (largest flow convergence, lowest TR velocity) and expiration (smallest flow convergence, highest TR velocity) should be performed (Fig. 5).29)
The intensity and the shape of TR velocity profile by continuous wave Doppler provides a clue to TR severity. The TR profile is generally parabolic except in cases with severe TR, where rapid equalization of RA and RV pressures results in a rapid deceleration of the Doppler tracing, also described as the "cut-off sign". Additional indirect clues of regurgitation severity are the size of RV and RA, the paradoxical interventricular septal motion, the systolic bulge of interatrial septum toward left atrium and the hepatic venous systolic flow reversal, which is a specific but relatively insensitive sign of severe TR.
In clinical practice, the echocardiographic assessment of FTR should integrate all signs and measurements obtained by all methods, especially when FTR is more than mild.19) However, it should be kept in mind that the degree of FTR is highly dependent on RV preload (i.e. intravascular volume status), afterload and RV systolic function. A reclassification of FTR severity at sequential examinations may only be the consequence of the optimization of diuretic treatment in heart failure patients. Moreover, there are no quantitative criteria for distinguishing between the mild and moderate degree of FTR. Finally, in routine clinical practice 2D PISA measurements of TR severity do not take into account the leaflet tethering (i.e. no angle correction is done), the non-rounded shape of the regurgitant orifice, as well as systolic and respiratory variations of regurgitant orifice and volume.
Since assessment of the severity of FTR has so many limitations, new approaches are being contemplated to select patients for surgery.
One has been proposed by Dreyfus et al.,17) instead of relying solely on TR severity, this novel approach takes into account to TA size, the mode of TV leaflet coaptation, and TV leaflet tethering factors modulated by RV enlargement and dysfunction.17) These parameters considered together may help in the decision-making with respect to the need for concomitant surgical TV annuloplasty and additional surgical repair techniques during left-sided heart valve surgery (particularly when TR is less than severe; Table 1).
Another approach is to a target that has more accurate quantification of TA. An increasing emphasis has been recently placed on the degree of TA dilatation as a trigger for TV repair, more than the actual severity of FTR. However, defining TA dilation is not so straightforward, and there is no standardized approach for its quantification by echocardiography.30) Since the TA is a dynamic structure with an asymmetric saddle shape, even small variations in the angle of the ultrasound beam or in the timing of measurements can result in considerable discrepancies in TA size.13) 2DE may underestimate the TA size, and 3DE probably offers a more accurate evaluation of TA dilation by its ability to yield quantitative measurements such as TA area, as well as maximal and minimal diameters, irrespective of their spatial orientation (Fig. 6).
Finally, another approach is to aim for a more accurate quantification of FTR severity by 3D color Doppler methods (3D PISA), which imply no geometric assumptions about the PISA and EROA shape. 3D PISA method was demonstrated to be feasible for clinical use and more accurate than 2D PISA method, even though the comparison was done against methods that could arguably considered as reference standards (3D color planimetry and quantitative 2D Doppler echocardiography based on tricuspid and pulmonary stroke volumes).31)
In the absence of specific contraindications, when echocardiographic imaging is limited by a suboptimal acoustic window, in patients with equivocal echocardiographic findings and when 3DE is not available for assessing RV volumes and function, cardiac magnetic resonance (CMR) is the technique of choice to assess patients with significant TR.32) CMR is not limited by an acoustic window and can image the whole heart in any plane providing excellent myocardial definition. CMR is presently the gold standard for the assessment of RV morphology and function, and accurate assessment of the RV is crucial to understand the underlying mechanisms and address management in patients with TR. TR jet can be qualitatively evaluated in a long-axis RV view by in-plane velocity mapping. The "signal void" phenomenon (corresponding to the turbulent regurgitant flow) and its extension into the RA can be used as a semi-quantitative parameter of TR severity by CMR. However, similarly to color Doppler jet area, this method is also influenced by RA pressure and by imaging parameters (i.e. echo time, flip angle, alignment with jet direction). In case of isolated TR, regurgitation volume can be calculated as the difference between the RV and LV stroke volumes. CMR has been also used to assess the configuration and dynamics of TV annulus;33)34) however CMR requires time-consuming acquisition and offline postprocessing for TA reconstruction from tomographic images, that is not practical for clinical use.
Multi-detector row cardiac computed tomography imaging (MDCT) is rarely only used for assessing the anatomy and function of TV, especially when echocardiography and CMR are either suboptimal or contraindicated. MDCT can help to understand the underlying mechanisms of FTR, as well as to assess right chambers size and function. Recent insights from MDCT have shown that the antero-posterior TA diameter, and not the septal-lateral TA diameter, was independently correlated with significant TR (>3+) in FTR patients, after adjusting for pulmonary systolic pressure and RV end-systolic volume.35) MDCT is faster and less hampered by metal artifacts than CMR, and can be particularly helpful for assessing patients with pacemakers/defibrillators. However, visualization of right-sided valves is less reliable than for the left-sided valves, due to their thinner structure and the fact that contrast injection protocols are not timed to enable visualization of the right heart.36) Protocols for right heart enhancement prolong the duration of contrast injection in comparison to a coronary computed tomography study.37) Radiation exposure, the lower temporal resolution and the inability to assess transvalvular flow and pressure gradients, as well as leaflet morphology are important drawbacks, favouring the use of echo (or CMR) for the routine TV workup.
Before the introduction of 2DE and Doppler echocardiography, cardiac catheterization was the main imaging modality to confirm the presence and severity of TR. The diagnosis of TR posed a greater challenge, as selective angiography into the RV would often distort the TV and catheter position in the RV would be difficult to maintain. The pressure waveform in the RA shows the characteristic prominent systolic V wave with rapid descent only in the most severe cases. At present, diagnostic cardiac catheterization should rarely, if ever, be undertaken for the diagnosis or quantitation of TV disease alone.
In the past, FTR was considered to progressively revert with time after effective surgical treatment of left-sided valves. Nevertheless, more recent studies showed that FTR could develop and evolve postoperatively to a significant degree over time in up to 40% of patients.38) Late FTR portends a poorer prognosis in terms of morbidity and mortality, and a high operative mortality in case of redo surgery.
After mitral valve surgery, significant FTR can develop after several years (up to 10 years or more). The most important risk factors associated with a higher probability of late FTR development involve clinical, echocardiographic and surgical factors (Table 2).
The presence of pulmonary hypertension is a significant risk factor for late TR, since pulmonary hypertension persists to some degree in approximately one-quarter of patients despite correction of the left-sided valve lesion.39) Therefore, pulmonary hypertension is a relative indication for TV repair, but only when coexisting with a dysfunctional TV (annular dilation and tethering, with any degree of FTR).
It should be said that treatment options in FTR are primarily surgical, either valve repair or replacement. Medical management allows only to control congestive symptoms in patients with RV failure (diuretics, ACE inhibitors) and in patients with chronic atrial fibrillation, to control the heart rate (digitalis) and reduce the thrombo-embolic risk (warfarin). In patients with pulmonary hypertension, pulmonary vasodilators may reduce TR severity.25)
The surgical treatment of FTR is still an object of debate in terms of indication, timing and surgical techniques.
Historically, a conservative approach has been the norm when dealing with FTR, with the assumption that "secondary" TR should resolve once the "primary" cause (typically mitral regurgitation or stenosis) is successfully treated. This approach continues to guide surgical practice to the present day, and TV annuloplasty is infrequently offerred to patients undergoing left-sided valve interventions in many surgical practices.40)41) More recent observational studies have demonstrated that uncorrected significant FTR during left-sided valve surgery can result in worse early and late outcomes due to progression of FTR and underlying right heart failure.42)43) Therefore, FTR should be treated at the time of left-sided valve surgery, as TV ring annuloplasty can be performed with minimal incremental morbidity and negligible additional mortality.
If the FTR is severe, a restrictive annuloplasty using a rigid prosthetic ring represents the most effective and durable method of treatment providing lower recurrence of significant TR and better long-term and event-free survival up to 15 years after surgery compared with suture annuloplasty (de Vega).44)45)46) Observational data suggest that more than 85% of patients undergoing tricuspid ring annuloplasty for FTR are free from moderate or severe insufficiency at their 10-year follow-up, compared with less than one-half of those patients who undergo isolated mitral valve surgery.44)
If the FTR is mild or moderate, the diameter of the TA rather than the grade of regurgitation (which is highly subjective and variable) should be the criterion to indicate the need for concomitant TV repair at the time of left-sided valve surgery.9) Compelling evidence show that, if the TA is dilated and is not corrected at the time of surgery, it is very likely that a significantly late TR will occur.47)48)49)50) For that reason, the 2012 European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS)9) and 2014 ACC/AHA25) guidelines strongly encourage the surgical correction of less than severe FTR if the TA dilation is greater than 40 mm (21 mm/m2) in patients undergoing left-sided valve surgery. However, in addition to TA dilation, also the degree of leaflet tethering predicts the development of late FTR. Therefore, the dimensions of the TA and the degree of tethering of TV leaflets should be systematically measured by echocardiography: in the presence of isolated annular dilatation, a restrictive ring annuloplasty should be performed; conversely, when leaflet tethering coexists with annular dilation, pericardial patch augmentation of the anterior leaflet and restrictive ring annuloplasty, or TV replacement should be considered (Table 1).
Suture and pericardial techniques have shown inferior repair durability than rigid annuloplasty.51) However, the success of a TV repair is also based on baseline patient factors. Risk factors for early (less than 6 months) TV repairs are the severity of preoperative TR and leaflet tethering. Risk factors for late failure (beyond 6 months) of TV repairs are: severe LV dysfunction, presence of permanent pacing and implantable cardioverter defibrillator wires that cross the TV, and repair type other than ring annuloplasty.
It should be acknowledged that the current evidence supporting these recommendations is rather limited, leaving room to personal experience-based rather than evidence-based surgical decision-making. It is not certain how much of the negative impact of FTR is causative, rather than confounding, and to what extent the performance of liberal "profilactic" tricuspid annuloplasty will improve long-term outcomes of mitral valve surgery. Most of the data concerning FTR after mitral valve surgery concern rheumatic and degenerative etiology, mainly in young or middle-aged patients; thus, at present there is little justification to extend the liberal use of TV annuloplasty to other etiologies, such as ischemic or endocarditis, or to elderly patients. Theoretically, more complications may result from a slightly prolonged operation, right atriotomy and the tricuspid annuloplasty, but the actual risk is difficult to quantify without a randomized clinical trial. Moreover, current techniques to repair FTR are associated with a significant degree of residual or recurrent regurgitation mainly because of failure to address all the components of this challenging entity. Prospective randomized trials are needed to booster the evidence behind the current recommendations.
Percutaneous treatment of FTR emerges as a potentially useful alternative for patients with FTR who are at high risk for conventional open heart surgery. In addition, there has been a considerable development in the percutaneous technologies for the mitral and aortic valves, which translate into a significant proportion of patients with concurrent FTR that would require non-surgical treatment options.
However, the development of percutaneous technologies for treating FTR is technically challenging, due to the peculiar anatomy of the TV and the incomplete understanding of the mechanisms/pathophysiology of FTR in different cardiac conditions (e.g. atrial fibrillation vs. pulmonary hypertension vs. RV overload, etc.).
The clinical experiments with percutaneous TV therapies are very preliminary and the principles of TV repair for each approach have been recently described.52)
Among the various treatment options undergoing clinical testing, those addressing the TA dilatation based on annuloplasty principle seem to be the most promising (Mitralign, TriCinch, TRAIPTA, etc.). However, the different approaches have different procedural risks (right coronary injury, leaflet injury, complete AV block, stent dislocation in very large inferior vena cava with TriCinch device, etc.) and the selection criteria (regarding both FTR pathophysiology and right heart anatomy) are currently unclear. It is foreseable that among the various imaging techniques, 3DE will play an important role in patient selection and in intraprocedural guidance, as well as in assessing the procedural outcome and complications; with some technically challenging devices (i.e. Mitralign, etc.), the use of 3DE for guiding the procedure appears crucial.
Several anatomic characteristics of TV pose a great challenge for the development of percutaneously deployable TV prostheses: the very large dimensions of TA in FTR (often exceeding 7 cm) make difficult to design a prosthesis that could be deployed through standard sheats and delivery catheters; angulation of TA with respect to inferior or superior vena cava (more difficult access); lower pressure and slow flow in the right heart chambers (higher risk of thombi, tissue overgrowth and calcifications, adversely affecting the durability of tricuspid prostheses); RV thin wall and trabeculated structure (risk of tamponade and device trapping in case of RV access). First experiences used Melody pulmonary prosthesis, SAPIEN and SAPIEN XT aortic valve prostheses adapted for the TV.53) Percutaneous therapies in development may eventually result in less invasive options and in earlier corrections of FTR than are currently offered.
Pathophysiology of FTR is complex (Fig. 1). However, we generally rely only on univocal interpretation of the pathophysiology of FTR: independent on the underlying cardiac condition, FTR is associated to TA dilation which is secondary to RV enlargement, and progression of RV and TA dilation results in lack of leaflet apposition starting a vicious circle of TR begetting more TR; further RV dilation and shape distortion determine TV leaflet tethering and aggravate TR severity.52)54) However, many patients with atrial fibrillation show significant TR despite a normal RV. The role of the RA in the development of FTR remains to be fully understood. Finally, patients with FTR in the setting of RV infarction may have a severe distortion of RV shape and papillary muscles without significant dilation of the RV cavity (at least during the early stages of TR). Accordingly, the involvement of the various components of TV apparatus in the development of FTR may vary with the underlying etiology, and it is likely that a tailored treatment strategy (surgical or interventional) is required for each etiology.
It is generally agreed that echocardiography is the reference technique to assess patients with FTR.17)54)55) However, the 2DE parameters routinely used to assess the size of the RV and TA (RV diameters and areas, TA diameter) are only rough estimates of the geometrical complexity of the RV and the TA. 3DE allowing a comprehensive visualization of the right heart structures holds promise for a more accurate, quantitative assessment of the RV, RA and TV apparatus. However, dedicated software packages specifically designed to account for the peculiar geometry and function of these structures are greatly needed.
Assessment of FTR severity is based on 2D and Doppler echocardiography concepts that have been developed for the mitral valve,19) without taking into account the peculiarities of the TV (three leaflets, only rarely visualized simultaneously in a single tomographic plane; complex regurgitant orifice shape; large systolic, respiratory and load-dependent variations in regurgitant orifice size) which make the assumptions of the PISA and vena contracta methods not applicable for this valve. New parameters able to accurately quantitate the severity of TR are definitely needed in order to better define the natural history of FTR and select patients who may benefit from surgical or interventional procedures.
Finally, all recommendations on the management of valvular heart disease in the 2012 ESC/EACTS and 2014 ACC/AHA guidelines concerning the indication for the surgical treatment of FTR are based on expert opinion only.9)25) We definitely need prospective outcome studies and registries to specifically address the indications for FTR treatment.
Current knowledge about FTR is that this valve disease is not truly functional, since it is secondary to anatomical abnormalities of a TV apparatus, such as dilation and change in shape of TA, papillary muscle displacement, and leaflet remodelling and tethering. The changes affecting the different components of the TV apparatus may vary according to the underlying cause of FTR development. Accordingly, the term "functional" should be replaced with "secondary".
Echocardiography remains the main imaging modality to assess both TR mechanism and severity, and 3DE will probably become the reference standard for these purposes. However, we need to change our paradigms and move forward from adapting the concepts and algorithms developed for the mitral valve to the TV, in order to gather TV-specific knowledge and design analysis tools accounting for both the anatomical and functional peculiarities of FTR. A better understanding of the regurgitation mechanisms in the different conditions at risk of developing significant FTR will allow a tailored selection of patients to refer to surgery or percutaneous procedures and optimize their timing.
In addition to surgical annuloplasty (with or without leaflet elongation), several percutaneous devices are being developed to offer a treatment option to high-risk patients. Imaging will play a key role to select the right patient for the most effective device and to guide the procedure.
Figures and Tables
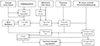 | Fig. 1Current perspectives on pathophysiology of functional or secondary tricuspid regurgitation. RV: right ventricular. |
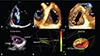 | Fig. 2Comprehensive assessment of the anatomy of functional tricuspid regurgitation by transthoracic three-dimensional echocardiography. Two-dimensional color Doppler showing severe functional tricuspid regurgitation (A). En face view of the tricuspid valve leaflets and annulus from the right atrial perspective (B). En face view of the tricuspid valve leaflets from the right ventricular perspective (C). Semi-automated quantitative analysis of tricuspid annulus size and shape, showing the relationship with the 4-chamber view plane (green line along the septal-lateral direction; D). Lateral view showing the flattening of tricuspid annulus (E). Surface rendering and quantitative analysis of tricuspid annulus and leaflets (F). A: anterior tricuspid leaflet, Ao: aorta, IVC: inferior vena cava, MV: mitral valve, P: posterior tricuspid leaflet, RVOT: right ventricular outflow tract, S: septal tricuspid leaflet. |
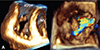 | Fig. 3Three-dimensional echocardiographic visualization of the complex geometry of the regurgitant orifice in functional tricuspid regurgitation. Volume rendering of the tricuspid valve at mid-systole from the ventricular perspective showing the complex star-shaped regurgitant orifice in both volume rendering (A) and color Doppler (B) display modes. RVOT: right ventricular outflow tract, A: anterior tricuspid leaflet, P: posterior tricuspid leaflet, S: septal tricuspid leaflet, MV: mitral valve. |
 | Fig. 4En face view of the regurgitant orifice in a patient with severe functional tricuspid regurgitation, illustrating which explains the limitations of 2D diameters in estimating the size of the regurgitant orifice. The complex star-shaped regurgitant orifice by 3D color Doppler imaging is displayed along with the two longitudinal cut planes of the regurgitant jet in apical 4-chamber (yellow line) and its orthogonal view (white line) (A). Vena contracta measured in B (corresponding to the apical 4-chamber view) is significantly smaller than in C (corresponding to the white line in A). None of them is aligned with the largest orifice diameter, which is oriented in antero-posterior direction (D). A: anterior tricuspid leaflet, P: posterior tricuspid leaflet, S: septal tricuspid leaflet. |
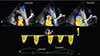 | Fig. 5Challenges in assessing the tricuspid regurgitation (TR) severity by two-dimensional-Doppler Proximal Isovelocity Surface Area method in a patient with severe functional TR in atrial fibrillation. Both color Doppler images (A to C) and continuous-wave Doppler tracings of regurgitant jet (D) show high respiratory and beat-to beat variability, stressing the need to average measurements from several beats performed during inspiration (flat flow convergence with large vena contracta diameter, lowest TR velocity) and expiration (more rounded flow convergence, narrower vena contracta diameter, highest TR velocity) to provide an average severity over the respiratory cycle. |
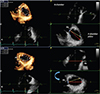 | Fig. 6Impact of various methods of tricuspid annulus diameter sizing, demonstrated by three-dimensional echocardiography. The volume rendering of the tricuspid valve anatomy is shown in correspondence with the orientations of different annulus diameters. The tricuspid annulus displayed in the apical 4-chamber view (upper right image) is 39 mm (blue diameter in the lower right image), and is significantly smaller than the maximal diameter (red arrow=54 mm) and antero-posterior inter-commissural diameter (yellow arrow=48 mm) (A). The dataset has been rotated counterclockwise so that the upper right image displays the maximal annular diameter; in this case, the upper right image is no longer a 4-chamber view, becoming more similar with the conventional parasternal short-axis view of tricuspid valve (B). The diameter measured in 4-chamber view significantly underestimates both antero-posterior and maximal tricuspid annulus diameters. Ao: aorta, A: anterior tricuspid leaflet, P: posterior tricuspid leaflet, S: septal tricuspid leaflet. |
Table 1
Management-oriented classification of functional tricuspid regurgitation

*No leaflet tethering (<8 mm). †Leaflet tethering may be present (≥8 mm). ‡If leaflet tethering is present. TR: tricuspid regurgitation. Modified from Dreyfus et al.17)
Acknowledgments
Dr. Eena Surkova has received a research grant from the European Association of Cardiovascular Imaging.
References
1. Braunwald NS, Ross J Jr, Morrow AG. Conservative management of tricuspid regurgitation in patients undergoing mitral valve replacement. Circulation. 1967; 35:4 Suppl. I63–I69.
2. Sade RM, Castaneda AR. The dispensable right ventricle. Surgery. 1975; 77:624–631.
3. Kim JB, Yoo DG, Kim GS, et al. Mild-to-moderate functional tricuspid regurgitation in patients undergoing valve replacement for rheumatic mitral disease: the influence of tricuspid valve repair on clinical and echocardiographic outcomes. Heart. 2012; 98:24–30.
4. Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004; 43:405–409.
5. Shiran A, Sagie A. Tricuspid regurgitation in mitral valve disease incidence, prognostic implications, mechanism, and management. J Am Coll Cardiol. 2009; 53:401–408.
6. Jeong DS, Park PW, Mwambu TP, et al. Tricuspid reoperation after left-sided rheumatic valve operations. Ann Thorac Surg. 2013; 95:2007–2013.
7. Kim YI, Kwon DA, Kim HK, et al. Determinants of surgical outcome in patients with isolated tricuspid regurgitation. Circulation. 2009; 120:1672–1678.
8. Zhu TY, Wang JG, Meng X. Does concomitant tricuspid annuloplasty increase perioperative mortality and morbidity when correcting left-sided valve disease? Interact Cardiovasc Thorac Surg. 2015; 20:114–118.
9. Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC). European Association for Cardio-Thoracic Surgery (EACTS). Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012; 33:2451–2496.
10. Sutton JP 3rd, Ho SY, Vogel M, Anderson RH. Is the morphologically right atrioventricular valve tricuspid? J Heart Valve Dis. 1995; 4:571–575.
11. Victor S, Nayak VM. The tricuspid valve is bicuspid. J Heart Valve Dis. 1994; 3:27–36.
12. Basso C, Muraru D, Badano LP, Thiene G. Anatomy and pathology of right-sided atrioventricular and semilunar valves. In : Rajamannan N, editor. Cardiac Valvular Medicine. London: Springer-Verlag;2013. p. 211–221.
13. Miglioranza MH, Mihăilă S, Muraru D, Cucchini U, Iliceto S, Badano LP. Dynamic changes in tricuspid annular diameter measurement in relation to the echocardiographic view and timing during the cardiac cycle. J Am Soc Echocardiogr. 2015; 28:226–235.
14. Fukuda S, Saracino G, Matsumara Y, et al. Three-dimensional geometry of the tricuspid annulus in healthy subjects and in patients with functional tricuspid regurgitation: a real-time, 3-dimensional echocardiographic study. Circulation. 2006; 114:1 Suppl. I492–I498.
15. Ton-Nu TT, Levine RA, Handschumacher MD, et al. Geometric determinants of functional tricuspid regurgitation: insights from 3-dimensional echocardiography. Circulation. 2006; 114:143–149.
16. Badano LP, Muraru D, Enriquez-Sarano M. Assessment of functional tricuspid regurgitation. Eur Heart J. 2013; 34:1875–1885.
17. Dreyfus GD, Martin RP, Chan KM, Dulguerov F, Alexandrescu C. Functional tricuspid regurgitation: a need to revise our understanding. J Am Coll Cardiol. 2015; 65:2331–2336.
18. Rogers JH, Bolling SF. The tricuspid valve: current perspective and evolving management of tricuspid regurgitation. Circulation. 2009; 119:2718–2725.
19. Lancellotti P, Moura L, Pierard LA, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve diseases). Eur J Echocardiogr. 2010; 11:307–332.
20. Singh JP, Evans JC, Levy D, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol. 1999; 83:897–902.
21. Badano LP, Agricola E, Perez de Isla L, Gianfagna P, Zamorano JL. Evaluation of the tricuspid valve morphology and function by transthoracic real-time three-dimensional echocardiography. Eur J Echocardiogr. 2009; 10:477–484.
22. Muraru D, Badano LP. Assessment of tricuspid valve morphology and function. In : Badano LP, Lang RM, Zamorano JL, editors. Textbook of real-time three dimensional echocardiography. London: Springler;2011. p. 173–182.
23. Kim HK, Kim YJ, Park JS, et al. Determinants of the severity of functional tricuspid regurgitation. Am J Cardiol. 2006; 98:236–242.
24. Tribouilloy CM, Enriquez-Sarano M, Bailey KR, Tajik AJ, Seward JB. Quantification of tricuspid regurgitation by measuring the width of the vena contracta with Doppler color flow imaging: a clinical study. J Am Coll Cardiol. 2000; 36:472–478.
25. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014; 63:e57–e185.
26. Song JM, Jang MK, Choi YS, et al. The vena contracta in functional tricuspid regurgitation: a real-time three-dimensional color Doppler echocardiography study. J Am Soc Echocardiogr. 2011; 24:663–670.
27. Chen TE, Kwon SH, Enriquez-Sarano M, Wong BF, Mankad SV. Three-dimensional color Doppler echocardiographic quantification of tricuspid regurgitation orifice area: comparison with conventional two-dimensional measures. J Am Soc Echocardiogr. 2013; 26:1143–1152.
28. Mascherbauer J, Maurer G. The forgotten valve: lessons to be learned in tricuspid regurgitation. Eur Heart J. 2010; 31:2841–2843.
29. Topilsky Y, Tribouilloy C, Michelena HI, Pislaru S, Mahoney DW, Enriquez-Sarano M. Pathophysiology of tricuspid regurgitation: quantitative Doppler echocardiographic assessment of respiratory dependence. Circulation. 2010; 122:1505–1513.
30. Miglioranza MH, Mihăilă S, Muraru D, Cucchini U, Iliceto S, Badano LP. Variability of Tricuspid Annulus Diameter Measurement in Healthy Volunteers. JACC Cardiovasc Imaging. 2015; 8:864–866.
31. de Agustin JA, Viliani D, Vieira C, et al. Proximal isovelocity surface area by single-beat three-dimensional color Doppler echocardiography applied for tricuspid regurgitation quantification. J Am Soc Echocardiogr. 2013; 26:1063–1072.
32. Kim HK, Kim YJ, Park EA, et al. Assessment of hemodynamic effects of surgical correction of severe functional tricuspid regurgitation: cardiac magnetic resonance imaging study. Eur Heart J. 2010; 31:1520–1528.
33. Maffessanti F, Gripari P, Pontone G, et al. Three-dimensional dynamic assessment of tricuspid and mitral annuli using cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging. 2013; 14:986–995.
34. Anwar AM, Soliman OI, Nemes A, van Geuns RJ, Geleijnse ML, Ten Cate FJ. Value of assessment of tricuspid annulus: real-time three-dimensional echocardiography and magnetic resonance imaging. Int J Cardiovasc Imaging. 2007; 23:701–705.
35. van Rosendael PJ, Joyce E, Katsanos S, et al. Tricuspid valve remodelling in functional tricuspid regurgitation: multidetector row computed tomography insights. Eur Heart J Cardiovasc Imaging. 2016; 17:96–105.
36. Nasis A, Mottram PM, Cameron JD, Seneviratne SK. Current and evolving clinical applications of multidetector cardiac CT in assessment of structural heart disease. Radiology. 2013; 267:11–25.
37. Tops LF, Krishnàn SC, Schuijf JD, Schalij MJ, Bax JJ. Noncoronary applications of cardiac multidetector row computed tomography. JACC Cardiovascular imaging. 2008; 1:94–106.
38. Porter A, Shapira Y, Wurzel M, et al. Tricuspid regurgitation late after mitral valve replacement: clinical and echocardiographic evaluation. J Heart Valve Dis. 1999; 8:57–62.
39. De Bonis M, Lapenna E, Sorrentino F, et al. Evolution of tricuspid regurgitation after mitral valve repair for functional mitral regurgitation in dilated cardiomyopathy. Eur J Cardiothorac Surg. 2008; 33:600–606.
40. Di Mauro M, Bezante GP, Di Baldassarre A, et al. Functional tricuspid regurgitation: an underestimated issue. Int J Cardiol. 2013; 168:707–715.
41. Vassileva CM, Shabosky J, Boley T, Markwell S, Hazelrigg S. Tricuspid valve surgery: the past 10 years from the Nationwide Inpatient Sample (NIS) database. J Thorac Cardiovasc Surg. 2012; 143:1043–1049.
42. Di Mauro M, Bivona A, Iacò AL, et al. Mitral valve surgery for functional mitral regurgitation: prognostic role of tricuspid regurgitation. Eur J Cardiothorac Surg. 2009; 35:635–639. discussion 639-40.
43. Ruel M, Kulik A, Rubens FD, et al. Late incidence and determinants of reoperation in patients with prosthetic heart valves. Eur J Cardiothorac Surg. 2004; 25:364–370.
44. Tang GH, David TE, Singh SK, Maganti MD, Armstrong S, Borger MA. Tricuspid valve repair with an annuloplasty ring results in improved long-term outcomes. Circulation. 2006; 114:1 Suppl. I577–I581.
45. Rivera R, Duran E, Ajuria M. Carpentier's flexible ring versus De Vega's annuloplasty. A prospective randomized study. J Thorac Cardiovasc Surg. 1985; 89:196–203.
46. McCarthy PM, Bhudia SK, Rajeswaran J, et al. Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg. 2004; 127:674–685.
47. Dreyfus GD, Corbi PJ, Chan KM, Bahrami T. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg. 2005; 79:127–132.
48. Benedetto U, Melina G, Angeloni E, et al. Prophylactic tricuspid annuloplasty in patients with dilated tricuspid annulus undergoing mitral valve surgery. J Thorac Cardiovasc Surg. 2012; 143:632–638.
49. Navia JL, Brozzi NA, Klein AL, et al. Moderate tricuspid regurgitation with left-sided degenerative heart valve disease: to repair or not to repair? Ann Thorac Surg. 2012; 93:59–67. discussion 68-9.
50. Taramasso M, Maisano F, De Bonis M, et al. Prognostic impact and late evolution of untreated moderate (2/4+) functional tricuspid regurgitation in patients undergoing aortic valve replacement. J Card Surg. 2016; 31:9–14.
51. Starck CT, Kempfert J, Falk V. Tricuspid valve interventions: surgical techniques and outcomes. EuroIntervention. 2015; 11:Suppl W. W128–W132.
52. Taramasso M, Pozzoli A, Guidotti A, et al. Percutaneous tricuspid valve therapies: the new frontier. Eur Heart J. 2016; pii:ehv766. [Epub ahead of print].
53. Lauten A, Figulla HR. Tricuspid valve interventions in 2015. EuroIntervention. 2015; 11:Suppl W. W133–W136.
54. Arsalan M, Walther T, Smith RL 2nd, Grayburn PA. Tricuspid regurgitation diagnosis and treatment. Eur Heart J. 2015; pii: ehv487. [Epub ahead of print].
55. Tornos Mas P, Rodríguez-Palomares JF, Antunes MJ. Secondary tricuspid valve regurgitation: a forgotten entity. Heart. 2015; 101:1840–1848.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download