INTRODUCTION
Pollen-food allergy syndrome (PFAS) is an immunoglobulin E (IgE)-mediated allergic manifestation to fruits and vegetables due to cross-reactivity with prior sensitization to plant inhalant allergens. PFAS is an emerging public health issue, with a number of studies reporting an increased prevalence of pollen allergies over the last decade due to changes in atmospheric CO
2, climate, and pollen counts.
123 PFAS is the most common food allergy in adults, and the prevalence of PFAS varies in the literature from 5–8%.
45
PFAS, called oral allergy syndrome previously, was thought to be restricted to oropharyngeal symptoms; however, extr-aoral symptoms and systemic symptoms in PFAS have been reported.
678 Moreover, one study reported that 3% of patients experienced systemic reactions without oral symptoms, and 1.7% experienced anaphylaxis.
69 Because the systemic symptoms reported by researchers have increased and because symptoms are not limited to the oral cavity, the use of the term PFAS is more relevant than oral allergy syndrome:
10 This historical background has led to confusion among allergists concerning the diagnosis of PFAS. In a US study investigating the perception of PFAS, allergists estimated that 5–8% of patients with pollinosis had PFAS.
4 However, PFAS has been reported in 20–70% of patients with a pollen allergy, and anaphylaxis was reported in 1–2%, which is more prevalent than what allergists had estimated.
7811
Although the importance of PFAS is increasing and triggering foods differ from geographic regions and dietary habits, there are only a few studies on PFAS in Korea.
1213 The first nationwide study of PFAS in Korea recently reported that the prevalence of PFAS is 41.7% in Korean patients with pollinosis and that 8.9% of patients with PFAS manifest with anaphylaxis, which is a substantial proportion among patients with PFAS.
14
Several allergen components from plant foods are known to contribute to the development of anaphylaxis. Lipid transfer protein and cross-reactive carbohydrate determinants are known as pan-allergens of the plant, and they exhibit thermostability and resistance to proteolysis, which enables the food allergen to reach intestinal absorption in its intact form.
15 Although several studies on anaphylaxis-inducing allergenic components have been investigated, studies on detailed clinical manifestations and risk factor analysis of anaphylaxis in PFAS have been rarely conducted worldwide. Therefore, in this study, we investigated the clinical characteristics and risk factors of anaphylaxis in PFAS among Korean patients with pollinosis using data from a previous nationwide survey of PFAS in 2016.
14
MATERIALS AND METHODS
Study design and participants
The nationwide, cross-sectional study on PFAS was conducted in South Korea between March and December 2016.
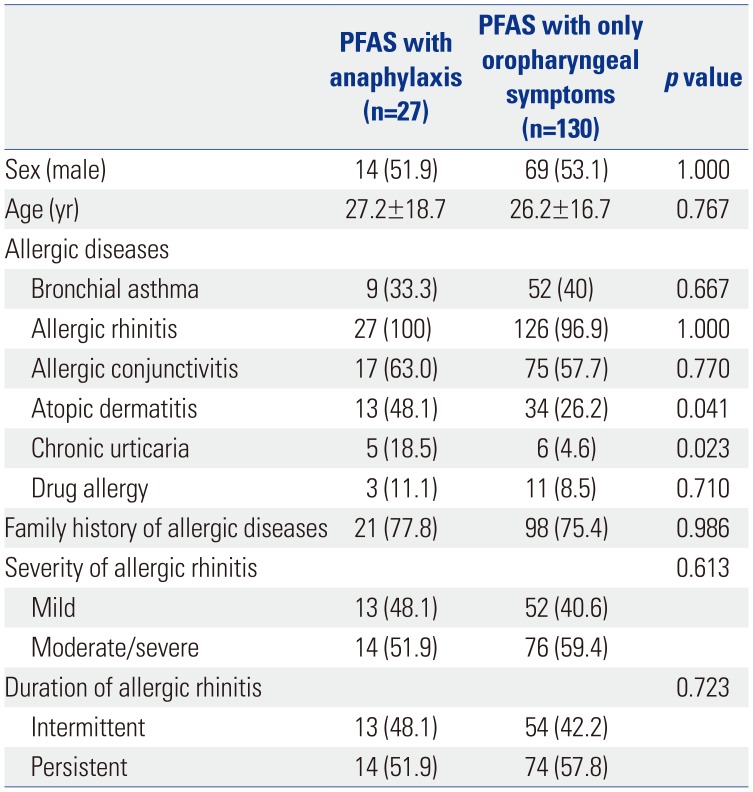
14 Data were collected from patients diagnosed with pollinosis at 21 institutes (19 university hospitals and two allergy clinics). The protocol was reviewed and approved by the institutional review boards of each institute (Hallym University Dongtan Sacred Heart Hospital, HDT-2016-04-155-003, etc.). Written informed consent was obtained from each patient or their parents.
Pollinosis was diagnosed according to 1) one or more allergic disease, including allergic rhinitis, allergic conjunctivitis, and/or bronchial asthma; 2) sensitization to the pollen of ≥one tree, grass, and/or weed; and 3) aggravated allergic symptoms when exposed to sensitized pollens. Sensitization to pollen was diagnosed by positive results to allergy skin tests and/or high serum-specific IgE levels using multiple allergen simultaneous tests [class ≥2+, Polycheck Allergy (Biocheck Co., Munster, Germany), AdvanSure Allergy Screen (LG Life Science, Seoul, Korea), AllergyScreen (Mediwiss Analytic GmbH, Moers, Germany)] or the ImmunoCAP® system (≥0.35 kU/L, ThermoFisher Scientific, Uppsala, Sweden). A positive skin prick test was defined as wheal size equal or greater to that of histamine [allergen/histamine (A/H) ratio ≥3+, Allergopharma (Reinbek, Germany), Lofarma (Milan, Italy), Bencard (Brefod, UK)] or a mean allergen wheal diameter of at least 3 mm.
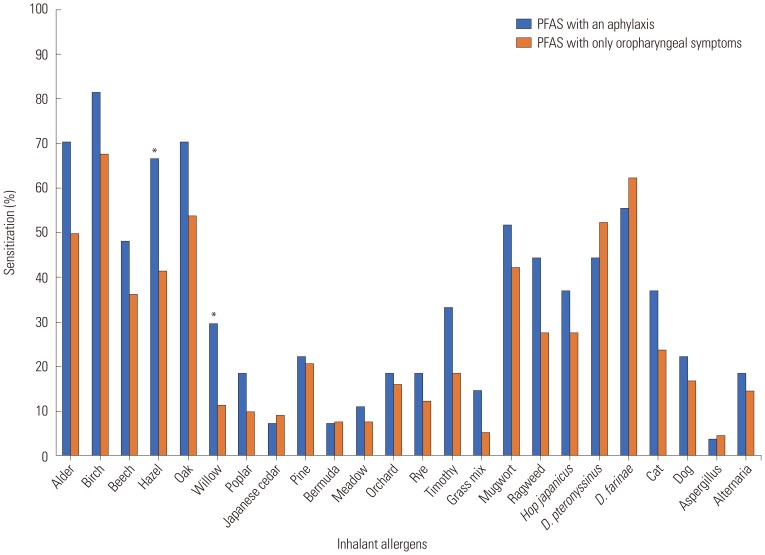
16 The investigated pollens included tree pollens (birch, alder, hazel, beech, oak, willow, poplar, pine, and tree mix), grass pollens (bermuda, meadow, orchard, rye, timothy, and grass mix), and weed pollens (mugwort, ragweed, and
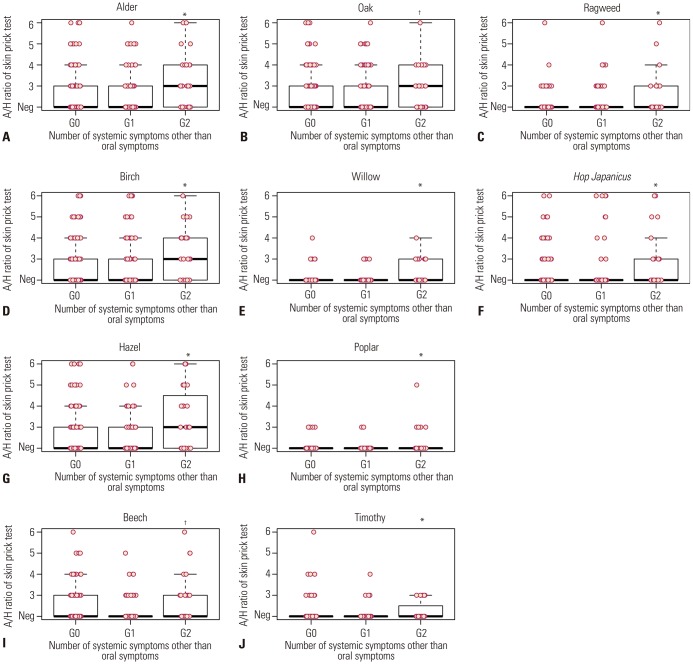
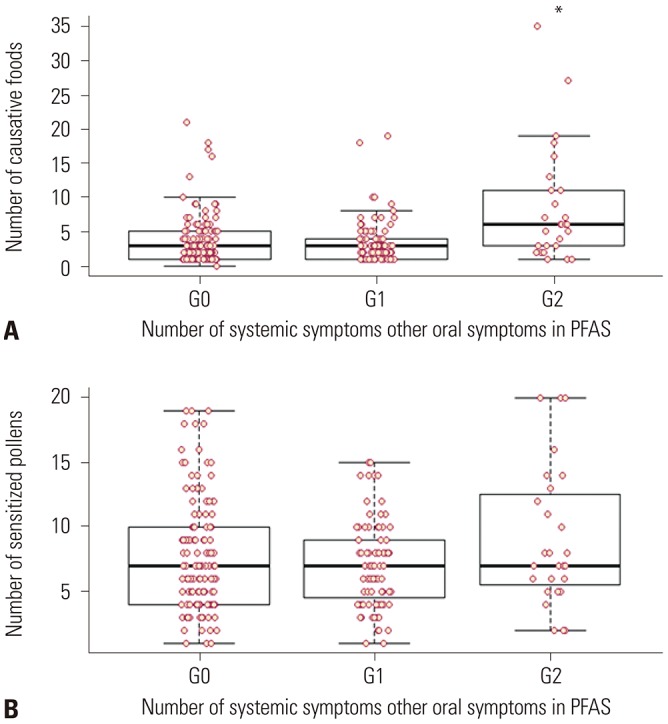
Hop Japanicus). Data were collected using a questionnaire and medical record review, including demographic characteristics, underlying allergic diseases, and allergy test results. For further analysis, we categorized subjects according to the number of systemic symptoms: 1) group 0 (G0), only oropharyngeal symptoms; 2) group 1 (G1), patients with any one systemic symptom; and 3) group 2 (G2) patients with more than two systemic symptoms, that is anaphylaxis.
Questionnaires about PFAS
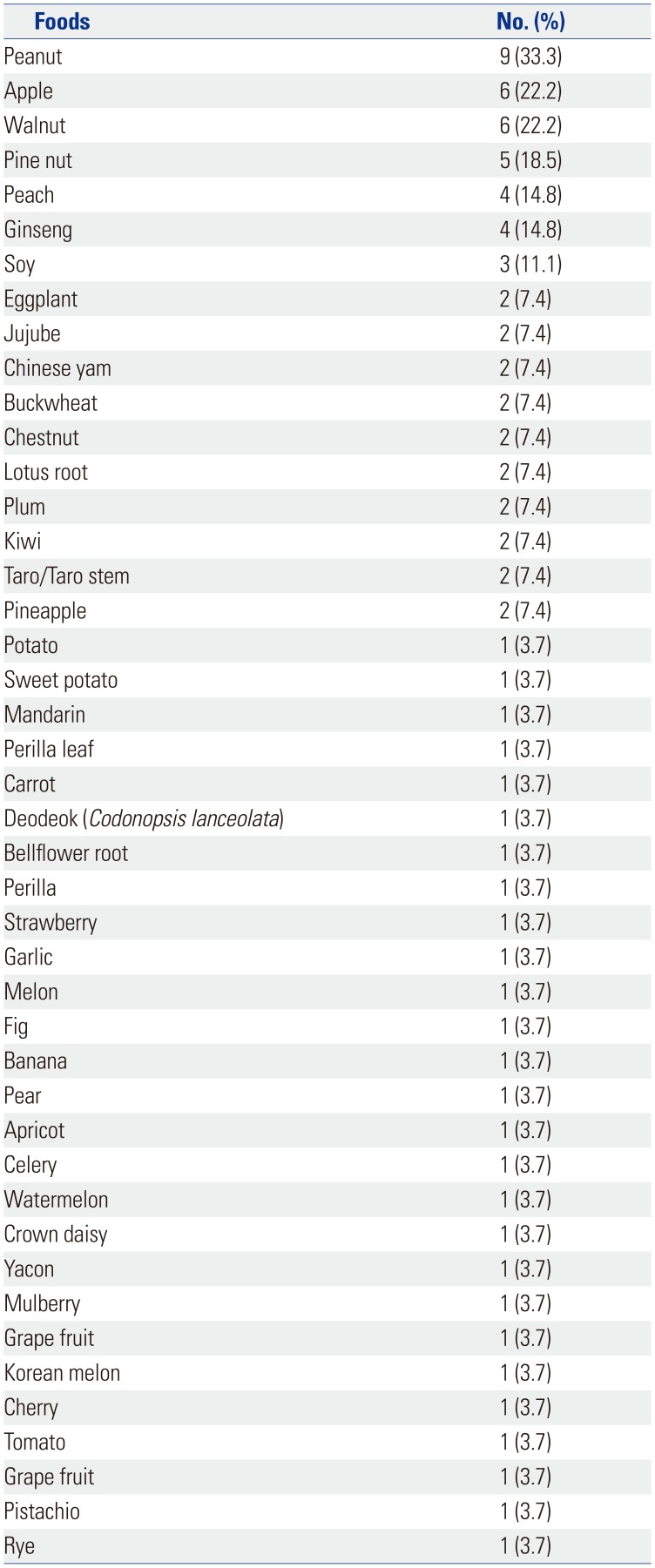
The list of culprit foods included apple, pear, peach, apricot, plum, cherry, watermelon, melon, Korean melon, banana, kiwi, orange, mandarin, pineapple, strawberry, mango, avocado, grape, carrot, potato, sweet potato, celery, crown daisy, perilla leaf, lettuce, kale, chicory, taro/taro stem, ginseng, deodeok (Codonopsis lanceolata), bellflower root, kudzu, lotus root, Chinese yam, eggplant, zucchini, cucumber, tomato, jujube, chestnut, peanut, walnut, pine nut, and soy.
In addition to oropharyngeal symptoms (tingling/itching sense or edema of the lips, oral cavity, and/or throat), systemic symptoms were categorized according to the organ in which the symptoms developed: dermatologic symptoms (itching, urticaria, or angioedema), respiratory symptoms (rhinorrhea, cough, dyspnea, wheezing, cyanosis, or hypoxia), cardiovascular symptoms (chest pain, hypotension, pale, sweating, or cardiac arrest), gastrointestinal symptoms (nausea or vomiting, diarrhea, or abdominal pain), neurologic and systemic symptoms (dizziness, unconsciousness, anxiety, change of sense, or death), and anaphylaxis. The diagnosis of anaphylaxis was confirmed by a physician using the criteria proposed in the second symposium on the definition and management of anaphylaxis by the National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network.
17 Anaphylaxis was defined by two or more of the following symptoms occur after exposure to a likely allergen, including involvement of skin tissue, respiratory compromise, cardiovascular symptoms, gastrointestinal symptoms, and neurologic symptoms.
Statistical analysis
All statistical analyses were performed using R version 3.4.0 (R Foundation, Vienna, Austria). Comparison between groups was performed using chi-squared analysis and Kruskal-Wallis test (>two independent groups) for discrete and continuous variables, respectively. Because the use of separate univariate tests leads to an inflated type 1 error, Bonferroni correction was applied to each pollen analysis. Multivariate logistic regression was utilized to determine potential predictors of anaphylaxis in PFAS. p<0.05 was considered statistically significant.
DISCUSSION
In this study, the most commonly offending foods of anaphylaxis in PFAS were peanut, followed by apple, walnut, pine nut, peach, ginseng, and soy in order of frequency. Anaphylaxis was significantly associated with the strength of sensitization to alder, birch, hazel, willow, timothy, and ragweed pollen. Presence of AD; sensitization to hazel, timothy, and ragweed; and the increased number of culprit foods for PFAS would more likely lead to the development of anaphylaxis in PFAS.
Approximately 20% to 70% of patients with pollinosis reported symptoms of PFAS after ingesting causative foods,
7811 and the prevalence of anaphylaxis in patients with PFAS is estimated to be 1–2%.
6 In our previous reports, the prevalences of PFAS and anaphylaxis in PFAS were 41.7% and 8.9% in Korea, respectively.
14 The prevalence of PFAS in our country was similar to that in previous reports for other countries, while the prevalence of anaphylaxis was much higher. This might be due to several reasons, such as 1) anaphylaxis in PFAS might be overlooked as class I food allergy by physicians; 2) the definition of anaphylaxis was broadly defined, including more than two organ involvements; and 3) the number of patients with PFAS might be increased when considering the increasing pollen allergic populations due to global climate changes and increases in pollen counts.
1718
In the present study, the most common anaphylaxis-triggering foods in PFAS were peanut, followed by apple, walnut, pine nut, peach, ginseng, and soy, whereas the most common causative foods of PFAS in Korea were peach, apple, kiwi, peanut, and plum.
14 Although common foods that are causative of anaphylaxis in PFAS have not been reported in other countries, apple and hazelnut are the most common causative foods of PFAS because of the high rate of birch sensitization in Europe, and apple and peach are known as the most common causative foods in relation to oak sensitization in Japan.
192021 In Korea, the most important sensitized pollens are oak, followed by birch and alder, which have cross-allergenicity with the Rosaceae family (apple, peach, plum, pear, cherry, apricot, almond, etc.), Apiaceae family (cantaloupe, honeydew, watermelon, zucchini, and cucumber), Fabaceae family (soybean and peanut), Juglans family (walnut), and Betulaceae family (hazelnut).
9182223 Accordingly, cross-reactivity patterns between pollens and foods vary depending on regional distribution of inhaled pollens and dietary habits. Relatedly, hazelnut anaphylaxis has not been reported in Korea, potentially in reflection of dietary customs of not consuming hazelnut, whereas ginseng is a frequently consumed herbal medication in Far-East Asia. Lim, et al.
24 reported a case of anaphylaxis after ingestion of fresh ginseng, wherein they found 17-kDa common allergens between birch pollen and ginseng using immunoblot analysis. Furthermore, Kim, et al.
25 reported that 45% of birch pollen-sensitized patients with allergic rhinitis show positive responses to skin tests with raw Korean ginseng extracts, suggesting cross-allergenicity between birch and ginseng. Interestingly, all of the anaphylaxis cases to ginseng were sensitized to birch pollen in our study. However, there has been no study about cross-allergenicity between pollens and other local foods, such as jujube, lotus root, crown daisy, yacon, and mulberry. Further research will be needed.
In this study, the most frequent causative food of anaphylaxis in PFAS was peanut. Sensitization to seed storage allergenic components (Ara h 1, 2, 3, and 6) in peanut suggests the possibility of class I food allergy, whereas isolated specific IgE to pathogenesis-related protein PR-10 family members, such as Bet v1 (birch), Mal d 1 (apple), Cor a 1 (hazelnut), Gly m 1(soybean), or Ara h 8 (peanut), usually causes PFAS without systemic symptoms.
26 Lipid transfer proteins, including Art v 3 (mugwort), Ara h 9 (peanut), Pru p 3 (peach), Mal d 3 (apple), and Gly m 1 (soybean), frequently are attributed to the development of anaphylaxis in PFAS.
27 However, this was a questionnaire-based epidemiological study, and allergenic components analysis was not performed. Ara h 9 (peanut) or other unknown allergenic components might contribute to the development of anaphylaxis in our study subjects. Further studies will be needed to elucidate which allergenic components of pollens and foods are related to the development of anaphylaxis in PFAS in these study subjects.
The risk factors of anaphylactic reactions in PFAS have been previously reported, such as systemic reactions to one of the associated foods and positive skin test results with commercial extracts.
28 In our study, the strength of sensitization to skin tests to alder, birch, hazel, willow, timothy, and ragweed was associated with development of anaphylaxis in PFAS. Furthermore, the sensitization to specific pollens, such as hazel, timothy, and ragweed, was an independent risk factor of anaphylaxis in PFAS. Eriksson, et al.
29 have reported a positive correlation between the size of the skin reaction to birch pollen and the incidence of hypersensitivity to PFAS; however, the association between the strength of pollen sensitization and anaphylaxis in PFAS has not been reported to date. In this study, the presence of AD and a higher number of culprit foods were also important risk factors for anaphylaxis in PFAS. AD has been reported as an important risk factor in a study with recurrent anaphylaxis patients.
30 Although the exact mechanism has not been elucidated, researchers have suggested that patients with AD have more activated mast cells and basophil cells.
31
Currently, many clinicians maintain that PFAS does not cause anaphylaxis.
4 Considering our study results with a higher prevalence of anaphylaxis in PFAS than that which has been reported, clinicians must be aware of the potential risks generated by PFAS and consider this when making a decision for risk management. If pollinosis patients have multiple pollen-related food allergies and strong positive responses on skin tests to hazel, timothy, or ragweed, the culprit foods may be related to the development of anaphylaxis. In addition, if patients have systemic symptoms or an anaphylactic reaction, allergists should educate the patients to avoid potential culprit foods, and they should not hesitate to prescribe self-injectable epinephrine.
This study has several limitations. First, this study was conducted as a questionnaire-based study depending on the patient's recollection and clinical history. Double-blind, placebo-controlled food challenge (DBPCFC), a gold standard for the diagnosis of food allergy, was not performed for diagnosis of PFAS in this study. Although DBPCFC is the gold standard for diagnosis of food allergy, careful clinical history in patients with pollinosis could replace provocation tests for diagnosis of PFAS because the excipient and taste covering for DBPCFC may cause the loss of the allergenic properties. Moreover, component-resolved diagnosis has yet to be prevalent for the diagnosis of PFAS. Therefore, in this study, PFAS was defined by typical oropharyngeal symptoms, including systemic symptoms, with raw fruits and/or vegetables and positive results of allergy tests to pollens. Second, this study was conducted by voluntarily participating hospitals, and the possibility of selection bias cannot be excluded. Because most of the contributing hospitals are referral hospitals, the prevalence of PFAS and anaphylaxis was possibly higher due to the involvement of more severe pollinosis cases. Third, all investigators did not use same allergy skin test solutions for diagnosis of pollen allergy, which are not standardized between manufacturers. This might affect the results of strength of pollen sensitization. Fourth, the relationship between specific allergenic components and the risk of anaphylaxis has not been studied. Component-resolved diagnosis could also help differentiating class I food allergy and PFAS, especially in case of peanut allergy. Further studies are needed.
In conclusion, the most frequently associated foods with anaphylaxis in PFAS were peanut, apple, walnut, pine nut, peach, ginseng, and soy in Korea. The presence of AD, strong sensitization to specific pollens, such as hazel, timothy, and ragweed, and a higher number of pollen-related foods significantly increased the risk of anaphylaxis in patients with PFAS, compared to patients with only oropharyngeal symptoms. Allergists should inform their patients that pollen-related foods can cause anaphylaxis and prescribe self-injectable epinephrine to high-risk patients.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download