INTRODUCTION
Hepatic hydrothorax (HH) is a complication of advanced cirrhosis characterized by transudative pleural effusion in the absence of underlying cardiac or pulmonary disease, with an estimated rate of 5–10% in cirrhotic patients.
1 HH results from pathologic trans-diaphragmatic migration of ascitic fluid in patients with liver cirrhosis.
2 In one study, HH was right-sided in 70% of cases, left-sided in 18%, and bilateral in 12%.
1 Patients with minimal pleural effusion may be asymptomatic or have pulmonary symptoms of dyspnea, cough, chest discomfort, hypoxemia, or respiratory failure. They are prone to recurrent bouts of spontaneous bacterial pleuritis with or without concurrent spontaneous bacterial peritonitis. Ascites usually presents in HH; therefore, patients with HH have poor underlying liver function.
3 The development of HH represents progression to decompensated cirrhosis and warrants prompt consideration for liver transplantation (LT).
4
Although LT is the optimal treatment modality for HH, it has poor accessibility due to donor organ shortage.
5 Long-term management of HH using methods other than LT is challenging, due to the underlying poor liver function and lack of clinical trials supporting a satisfactory therapy. Medical therapy with diuretics, as well as a low-salt diet, repeated thoracentesis, pigtail drainage, transjugular intrahepatic portosystemic shunt (TIPS), and surgery have been reported for the management of HH.
6 However, no large-scale studies have determined the optimal treatment modality, other than LT, for patients with HH. Therefore, this study aimed to identify the clinical characteristics and outcomes of cirrhotic patients with HH undergoing various modalities, such as medical treatment, repeated thoracentesis, pigtail drainage, and surgery.
MATERIALS AND METHODS
Patients
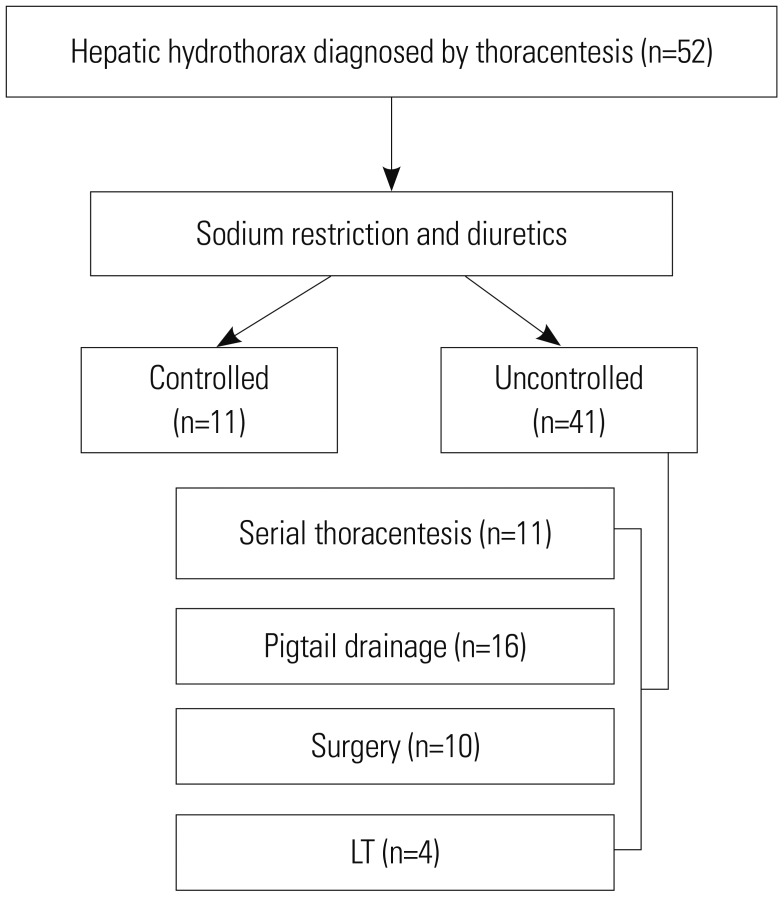
The inclusion criteria were age ≥18 years, diagnosis of liver cirrhosis, and refractory HH occurring between January 2013 and December 2017. Since surgery for HH has been available since 2013 at our center, we included all patients who were diagnosed with HH within the study period. HH was defined as large transudative pleural effusion (>500 mL) in a patient in whom other causes, such as heart failure, pneumonia, and malignancy, had been excluded. Data were collected from hospital records, and data on mortality and cause of death were obtained using the Statistics Korea microdata integrated service (
https://mdis.kostat.go.kr). The institutional review board committee of Chonnam National University Hospital (CNUH-2018-132) approved the study protocol, and the requirement for informed consent was waived as patient data were de-identified.
Pleural fluid analysis
All patients underwent more than one thoracentesis, and all samples of pleural fluid obtained initially were examined. Routine pleural fluid and serum examinations included cell counts with differential counts, gram stains, cultures, pleural fluid pH, serum and fluid protein levels, albumin level, bilirubin level, and lactate dehydrogenase (LDH) level.
Transudate pleural fluid was defined as having either a pleural fluid/serum protein ratio ≤0.5, a pleural fluid/serum LDH ratio ≤0.6, or a pleural fluid LDH level less than two-thirds of the upper limit of normal for serum LDH. Serum-pleural fluid analysis gradient was defined as the difference in albumin levels between serum and pleural fluid.
Treatment modalities
Patient groups were defined using four different treatment modalities. Serial thoracentesis group included patients who had at least more than two therapeutic thoracentesis without other treatment modalities and were on continuous diuretics therapy. The median time interval between consecutive thoracenteses was calculated in this group.
Pigtail drainage group included patients who had a pigtail catheter inserted using Seldinger technique and were on continuous diuretic therapy. Pigtail catheter was maintained for continuous drainage of pleural effusion, and re-insertion/removal was performed according to clinical requirements. Usually, thoracentesis was routinely performed before pigtail drainage for evaluation and initial management of hydrothorax. However, in case of massive pleural effusion that may not be managed with thoracentesis, initial drainage was achieved with pigtail drainage. If clinically needed, especially when a patient re-suffered from dyspnea after pigtail drainage removal, additional thoracentesis was performed. Pleurodesis was not performed in pigtail drainage group.
LT group included patients who underwent LT due to advanced liver cirrhosis combined with HH. Surgery group included patients who underwent surgery for the management of HH. All patients in surgery group underwent video-assisted thoracoscopic surgery (VATS) for the detection and closure of diaphragmatic defects (
Supplementary Fig. 1, only online) with concomitant pleurodesis. Pleurodesis was performed with Abnoba Viscum F (ABNOBA GmbH, Pforzheim, Germany) in eight patients, and Steritalc® (Novatech SA, La Ciotat, France) powder in two patients. A 24-Fr chest tube was maintained for 7–8 days and removed after confirmation of stable chest tube drainage volume. Diaphragmatic defect closure was performed using sutures and additional fibrin glue. Postoperative positive expiratory end pressure (5–10 cmH
2O) in an intubated state was maintained for 1 day, and peritoneal drainage for 5 days. Surgery for HH was considered successful if the patient had no recurrence of ipsilateral pleural effusion, or if they did not exhibit symptoms requiring drainage in case of recurrence. Surgery for HH was considered to have failed if there was recurrence of pleural effusion requiring drainage. After discharge, plain chest radiographs were obtained every 2 or 3 months in outpatient clinics to check for recurrence.
Patients with refractory HH were recommended to undergo LT. Consultations at specific departments were arranged for detailed counselling.
Definition and management of refractory HH
HH was diagnosed using the following international criteria: 1) serum-to-pleural fluid albumin gradient >1.1; 2) pleural fluid total protein <2.5 g/dL or pleural fluid/serum total protein ratio <0.5; 3) pleural fluid/serum LDH ratio <0.6; and 4) polymorphonuclear cell count <250 cells/mm
3.
7 Refractory HH was defined as pleural effusion that 1) failed to respond to restriction of salt intake, the maximum dose of diuretic treatment (spironolactone at 400 mg/day and furosemide at 160 mg/day), and serial thoracentesis of more than two times; or 2) reappeared rapidly after therapeutic thoracentesis.
The number of needle punctures for drainage per person-years and the amount of drainage per day were analyzed. The occurrence rates of acute kidney injury (AKI) and hepatorenal syndrome (HRS) were recorded. AKI was defined as an increase in serum creatinine level of ≥0.3 mg/dL within 48 h, or an increase in serum creatinine to ≥1.5 times the baseline level. HRS was defined as occurrence of AKI in patients despite diuretic cessation and albumin infusion, and chronic or acute hepatic disease with advanced hepatic failure and without any other apparent cause for AKI.
Ascites grading
Grading of ascites amount was based on the quantitative criteria proposed by the International Ascites club.
8 Grade 1 ascites is mild and can be detected only by imaging examination, such as ultrasonography. Grade 2 ascites is moderate and evidenced by moderate symmetrical distension of the abdomen, and is therefore readily detectable on physical examination. Grade 3 ascites is large with marked distension of the abdomen.
Statistical analysis
Data are expressed as mean±standard deviation, or median with range. Chi-squared test or Student's t-test was used for univariate analyses, and logistic regression was used for multivariate analyses. Analysis of variance was used for multifactorial comparisons. Cumulative overall survival was calculated using Kaplan-Meier method and compared between groups using log-rank test. Variables with p<0.05 in univariate analysis were entered into multivariate Cox regression analysis. In all analyses, p<0.05 was considered statistically significant. All statistical analyses were conducted using Statistical Package for the Social Sciences for Windows version 20.0 (IBM Corp., Armonk, NY, USA).
DISCUSSION
This study demonstrated the characteristics and clinical outcomes of patients treated with four different modalities for the management of HH. Some previous studies have reported on the results of various modalities for HH treatment;
91011 however, these studies were limited to a few methods. To the best of our knowledge, this is the first study to elucidate and compare the safety and efficacy of surgery and pigtail drainage, representing refractory HH patients with decompensated cirrhosis encountered in clinical practice.
Regarding risk factors for HH, alcohol consumption was the most common cause of HH in the present study. Chronic alcohol consumption and poor calorie intake causes muscle atrophy, resulting in low BMI and cachexia. These factors may increase the risk of anatomic thinning and the separation of collagenous fibers in the tendinous portion of the diaphragm.
12
Regarding treatment modality other than LT, many management options are available, such as serial thoracentesis, pigtail drainage, chest tube insertion, TIPS, and surgery. However, most patients have severely decompensated liver cirrhosis with poor liver function. Therefore, many difficulties are associated with making treatment decisions in clinical practice.
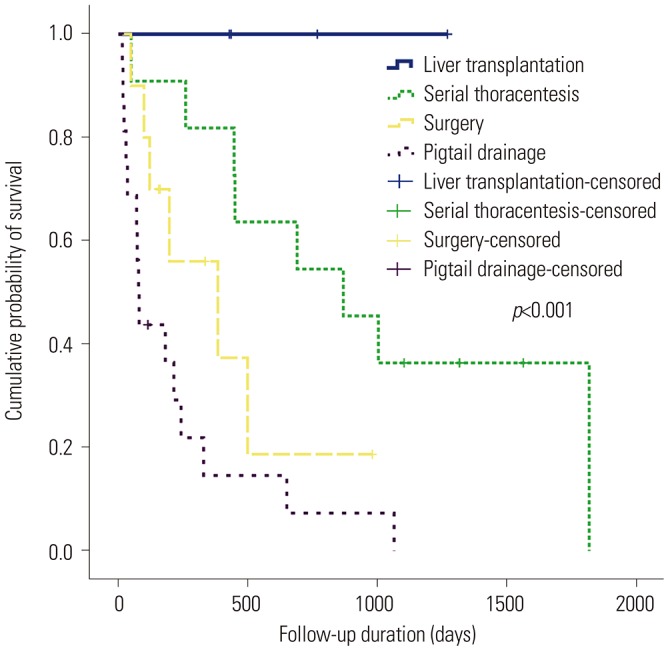
In terms of clinical outcomes based on treatment modalities, LT had the most favorable outcomes. Although the total number of patients who underwent LT was small, all four patients survived, with median survival duration of 601.5 days. Consistent with other studies,
911 patients who underwent serial thoracentesis showed better survival than those who underwent pigtail drainage or surgery in our study. Unlike other studies that did not consider the liver function of patients in each group,
911 serial thoracentesis group had better liver function than the other two groups (pigtail drainage and surgery) in the current study. This better liver function, rather than treatment modality, might have led to the higher survival rate. Therefore, considering the impracticality of LT in clinical practice, serial thoracentesis on demand can be an option for patients with relatively preserved liver function. However, serial thoracentesis was not effective in some patients with more advanced cirrhosis; and in these cases, pigtail drainage or surgery was performed in the present study.
Traditionally, surgery for HH is considered to have high risks for morbidity and mortality due to portal hypertension and poor liver function.
13 Although there have been several studies concerning the surgical management of HH, these studies are limited and clinical outcomes were not positive.
1415161718 However, the results of the present study showed a success rate of 80.0% without recurrence and surgery-related mortality. Furthermore, compared to pigtail drainage, needle puncture was required less frequently, and the total drainage amount of pleural and peritoneal fluid was smaller (9.3 times vs. 23.5 times, 125.5 mL/day vs. 288 mL/day, respectively). However, paracentesis was required more frequently in surgery group as per the main mechanism of HH (the passage of ascites via diaphragmatic defects), and the distribution of ascitic fluid between peritoneal and thoracic cavities became confined to peritoneal cavity only, increasing the occurrence rate of ascites.
5141920212223 For these reasons, the obliteration of diaphragmatic defects can eventually increase the amount of ascitic fluid. However, despite evaluating the dose of diuretics, AKI and HRS occurred more frequently in pigtail drainage group than in surgery group, although statistically not significant (
p=0.548,
p=0.432, respectively). Kaplan-Meier analysis revealed that the cumulative overall survival was longer in surgery group than in pigtail drainage group (402.1 days vs. 221.7 days,
p=0.113). However, this superior result of surgery group may be due to selection bias that patients in surgery group may have had more preserved liver function and better performance score than those in pigtail drainage group. Although it was not statistically significant, both CPT score and MELD score was lower in surgery group.
Furthermore, we compared survival rates between patients with CTP scores >10 and those with CTP scores ≤10. CTP >10 group had poorer survival rate than CTP ≤10 group. This suggests that in patients who are ineligible for LT, considering its ability to provide better quality of life and non-inferior survival duration compared to pigtail drainage, surgery can be an option in patients with refractory HH and CTP ≤10. No significant difference in the causes of death was found between the two groups. Although no life-threatening events occurred, various adverse events, such as chest tube site oozing, bleeding, hernia due to increased peritoneal pressure, infections, and pain, occurred in both groups.
TIPS is recommended in several studies and guidelines for managing HH, and the following criteria for TIPS have been suggested: age <65 years, MELD <18, CTP class A or B, cardiac ejection fraction >60%, no history of severe encephalopathy, no response to diuretic treatment, and/or repetitive therapeutic thoracentesis.
132021242526 However, these criteria in refractory HH patients have a narrow spectrum due to poor liver function, and their application in practice is challenging. The patients enrolled in this study had a median MELD score of 19, and 70.7% of patients had CTP class C. Furthermore, 29.3% of patients had a history of severe hepatic encephalopathy (>grade 2). For these reasons, TIPS was not considered a treatment modality in most cases of refractory HH in the present study.
In multivariate analysis for survival, BMI <19 kg/m
2, HH managed with pigtail drainage, and CTP score >10 were associated with poor survival rate. Patients with liver cirrhosis exhibit progressive loss of fat and muscle mass. Severe loss of muscle and body mass are known to be related to poor prognoses.
27 In this study, cachexia with low BMI (<19 kg/m
2) was the strongest factor associated with survival rate (hazard ratio 10.6,
p=0.002). Therefore, encouraging cirrhosis patients with low BMI to gain body weight and providing them with nutritional intervention should be considered in clinical practice. CTP score, which reflects the grade of ascites and encephalopathy, was related to survival rates, rather than MELD score.
This study had several limitations. First, this was a single-center retrospective study based on hospital records and locoregional patient information. Second, the number of included patients was small, due to the short survival duration of decompensated liver cirrhosis patients and low incidence rate of HH. Considering the small sample size and selection bias, which may have occurred when deciding the treatment modality for various patient conditions, these factors may have influenced the clinical outcomes of treatment modalities and may be impetuous to draw a conclusion. Therefore, further studies investigating a large number of patients with hydrothorax are required.
In conclusion, serial thoracentesis may be recommended for management of HH. In patients who have refractory HH not managed with serial thoracentesis, surgical management can be a useful option, alternative to pigtail drainage despite several concerns. Further studies investigating a large number of patients with hydrothorax are required.








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download