Abstract
Hepatic artery complications after liver transplantation include hepatic artery thrombosis, stenosis, pseudoaneurysm (PA), and others. Among these complications, hepatic artery PA is reported to have a low incidence, but it is associated with a devastating and often fatal outcome and a high risk of rupture. Herein, we present the case of a 56-year-old male patient who underwent living-donor liver transplantation because of alcoholic liver cirrhosis. A silent PA of the hepatic artery proper was detected incidentally on computed tomography scan during routine follow-up 15 days after surgery, and was successfully excluded by implantation of a coronary balloon-expandable stent-graft using ‘conical remodeling’ by two-step ballooning. This technique can be effectively used for PA of visceral arteries with tapering-small diameter, in which flow preservation is critical to patient care.
Hepatic artery pseudoaneurysm (PA) following liver transplantation (LT) is rare, with a reported incidence of 0.3–2.5%, but it can have devastating and often fatal consequences, with a high risk of rupture (12). Important contributing factors associated with extrahepatic PA include local infection (likely related to immunosuppression), biloma, biliary tract infection, and others (12). An extrahepatic PA is usually detected near the hepatic arterial anastomosis (12). The common clinical presentation of PA is rupture with massive hemorrhage. It may rupture into the peritoneum freely, the gastrointestinal tract, or the biliary tract. PA can lead to shock, gastrointestinal bleeding, hemobilia, decreased hemoglobin level, and abnormal liver function test results. The mortality rate associated with PA rupture has been documented to be as high as 78% (12).
Conventional treatment includes surgery, such as resection with re-anastomosis, reconstruction, and replantation; these techniques are technically difficult and are associated with morbidity and mortality. Recently, several reports have documented successful endovascular treatment, including coil embolization, stent, and stent-graft for PA following LT (34). Herein, we present a case of PA that developed within 1 month after living-donor LT, which was successfully treated using a coronary stent-graft with ‘conical remodeling’ by two step ballooning.
A 56-year-old male patient with alcoholic liver cirrhosis (Child-Pugh class B, with esophageal varix and refractory ascites) presented to our institution for living-donor LT. Laboratory values for liver function tests including serum bilirubin, aspartate transaminase (AST) and alanine transaminase (ALT) levels, were within the normal ranges. Intraoperatively, severe atherosclerotic changes of the recipient right hepatic artery were identified; thus, the recipient left hepatic artery was chosen for anastomosis of the donor right hepatic artery and the recipient right hepatic artery was ligated, leaving a short stump.
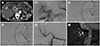
On postoperative day 15, a hepatic artery PA measuring approximately 1.7 cm in length, with a small amount of fluid collection along the hepatic resection margin, was incidentally detected during routine follow-up computed tomography (CT) (Fig. 1A). The PA was located on the posterior aspect of the proper hepatic artery, proximal to the anastomosis. The patient was asymptomatic and showed no signs of infection. Laboratory tests demonstrated a normal white blood cell count (8300/µL), no change in the hemoglobin level (8.4 g/dL), and normal levels of AST (16 U/L) and ALT (30 U/L). Hepatic angiography performed to determine the risk of potential rupture failed to identify the PA that was visualized on CT; thus, the patient was treated conservatively.
On postoperative day 23, repeat follow-up CT revealed a persistent PA along the proper hepatic artery with an enlarged fluid collection along the resection margin near the PA. Percutaneous catheter drainage was performed for the enlarged fluid collection to aspirate old blood, which showed no signs of infection. Repeat hepatic angiography with a catheter at the celiac trunk using a power injector showed indirect delayed contrast filling of the PA along the proper hepatic artery with small aneurysmal change of the ligated stump of the recipient right hepatic artery. Selective hepatic arteriography with the catheter at the distal proper hepatic artery using hand injection showed no contrast filling of the PA and failed to identify the rupture point (aneurysm neck) (Fig. 1B). We decided to implant a stent-graft because of the possibility of concealed rupture of the proper hepatic artery with minor leakage, although we could not identify the exact rupture site. A 6-F Ansel guiding sheath (Cook Medical, Bloomington, IN, USA) was placed within the common hepatic artery. Then, the aneurysmal stump and anastomosis site were crossed with a micro-catheter using a 0.014-inch guide wire (Transcend; Boston Scientific Corp., Watertown, MA, USA). During the procedure, the aneurysmal stump of the recipient right hepatic artery was incidentally ruptured, showing subtle contrast filling of the PA. The possibility of minor leakage from the hepatic artery proper itself was not ruled out, because of the absence of detectable filling of the PA on the selective arteriogram. The target artery for stenting showed a tapering shape on CT, with diameters of 3.3 mm at the proper hepatic artery proximal to the PA, and 2.7 mm just distal to the PA on angiography. The coronary balloon-expandable stent-graft (GraftMaster, Abbott Vascular, Santa Clara, CA, USA), 19 mm in length and with a maximum diameter of 4 mm, was placed over the 0.014-inch micro-guidewire from the proximal proper hepatic artery to the artery just distal to the anastomosis, covering the aneurysmal stump of the recipient right hepatic artery through the placed 6-F guiding sheath. The entire stent-graft was first expanded to 3.33 mm (oversizing by 20% to the distal target vessel) by ballooning until 15 atm (Fig. 1C). Thereafter, the deflated balloon was pulled back slightly proximal to the stent-graft, and then inflated up to 19 atm, making the diameter 3.95 mm (oversizing by 20%) for the proximal target vessel (conical remodeling) (Fig. 1D). Completion angiography after successful stent-graft placement showed complete exclusion of the ruptured stump of the recipient right hepatic artery and PA with hepatic arterial flow preservation (Fig. 1E). The patient's post-procedure course was uneventful, and laboratory findings were unremarkable. Routine follow-up CT 17 months later demonstrated good stent-graft patency without any evidence of PA (Fig. 1F).
In the current case, we did not detect any evidence of infection. Our patient had a normal white blood cell count and no fever. The fluid draining from the resection margin appeared to be old blood without signs of infection. The patient underwent duct-to-duct anastomosis for the bile duct connection during the initial operation, and there was no evidence of bile duct complication or cholangitis on CT. So the accurate cause of the PA was unclear, but severe atherosclerotic changes of the ligated native right hepatic artery might had caused minor arterial leak that was too slow flow with narrow breach to detect on angiography, resulting in false-negative findings of the PA on the first hepatic angiography.
There are many treatment options for PA. Conventional treatment consists of surgery, such as ligation and/or revascularization or retransplantation; however surgical treatments carry risks associated with repeat surgery, including those related to postoperative inflammation and adhesions that must be addressed during the re-operation; high rates of morbidity and mortality are reported (13). Since the 2000s, reports of successful endovascular treatment for PA have been increasing (34). Coil embolization can be used for PAs having an obvious narrow neck, but it carries a risk of fatal complications, including hepatic artery thrombosis or graft ischemia due to interruption of critical arterial flow to the graft (34). A PA possessing a defective vessel wall, which carries a risk of rupture during or after coil embolization, should be considered different from a true aneurysm.
The implantation of a stent-graft for PA is considered an ideal alternative endovascular treatment, as a stent-graft can exclude blood flow to the PA without sacrificing blood flow to the graft. Additionally, in the case of a failed stent-graft, patients have additional surgical options. Elias et al. (4) reported that a stent-graft will not affect surgical treatment following deployment. Also, a stent-graft might help the surgeon identify the location of the target artery in the adhesive fibrous tissues and prevent additional gross hemorrhage. Saad et al. (2) reported a high success rate (82%) of stent-graft placement for extrahepatic PA in patients after LT. Our patient was asymptomatic, and the post-operative period was sufficient for the development of adhesions; thus, we decided to use the minimal invasive endovascular technique for stent-graft implantation.
In visceral arteries, it is difficult to negotiate and place a guiding sheath with a large profile at the proper position and to deliver the stent-graft through tortuous and narrow arteries. The hepatic arteries frequently have small caliber, less than 4 mm, and a tapering arterial diameter, which can make the procedure difficult and technically complex. Several reports have proposed the use of coronary-based techniques and equipment in celiac and hepatic artery intervention in patients that are post-LT (5). Coronary-based stents or stent-grafts have more trackability and crossability for tortuous small caliber vessels. Hamby et al. (5) reported poor trackability with self-expandable stents and improved trackability with coronary stents for post-LT hepatic artery stenosis. We used a coronary balloon-expandable stent-graft because of the small diameter of the target hepatic artery.
Additionally, careful consideration of the optimal stent-graft diameter for the given vessel size is necessary. The best method to determine the appropriate diameter for treating a given vessel has not been well investigated. In the case of a tapering target vessel, the stent-graft diameter must be sized to the diameter of the larger proximal vessel, resulting in oversizing of the stent in relation to the diameter of the smaller distal vessel. According to the VIPER trial with Viabahn Endoprosthesis, the 1-year primary patency rate of stent-grafting was significantly higher (88%) when the stent-graft was oversized by < 20% than that when the stentgraft was oversized by > 20% (70%) (6). Undersizing of the stent-graft will create a gap between the graft and the wall, leading to inadequate apposition with the target vessel wall, perigraft flow (endoleak), and an increased risk of device migration. Amblard et al. (7) proposed that oversizing of the stent-graft by at least 20% may help prevent type I endoleak in stented abdominal aortic aneurysm. Lu et al. (8) reported their successful experience using conical remodeling of the balloon-expendable stent-graft. In their report, hepatic artery PA after LT was initially treated with a balloon-expandable stent-graft, but delayed endoleak was detected on follow-up CT 6 days after the procedure, which was not detected at the end of the procedure because of underdilation of the stent-graft. They remodeled the previously deployed stent-graft conically using two steps of balloon angioplasty for apposition to the target vessel with a tapering course, instead of deployment of an additional stent-graft. In the present case, we planned two-step balloon remodeling of the stent-graft ahead of the procedure, because the target hepatic artery showed a tapering course. Also, we performed stent-graft implantation with oversizing by 20% to both the proximal and distal target vessels in order to adequately approximate the vessel wall. Determining therapeutic strategy for angiographically occult visceral artery PA (invisible PA on catheter angiography), stent-graft placement would be good choice, because embolization is practically impossible due to invisible target lesion. Some reports have also demonstrated ultrasound-guided direct thrombin injection in case of angiographically occult visceral artery PA with good results (910). However, it could have challenges for hepatic artery to delineate the accurate lesion with ultrasound, because it would have difficulties including bowel gas shadowing in front of target interfering with lesion detection, close proximity to risk structures such as major blood vessels, and potential risk of native artery thrombosis.
The limitation of the present case is difficulty of comparing between this conical remodeled stent-grafting and simple cylinder shaped stent-grafting in terms of endoleak, and long-term patency, because of contributing factors. The diameter, tortuosity of target vessel, and technical feasibility can influence in outcome.
In conclusion, when a stent-graft is used to treat hepatic artery PA following living-donor LT, technical considerations, including the small caliber of the hepatic artery, tapering arterial diameter, and vascular tortuosity should be estimated. The use of a balloon-expandable coronary stent-graft with conical remodeling can be effective for PA of visceral arteries with tapering-small diameter, in which flow preservation is critical to patient care, and can show good follow-up patency. Much more research about the effectiveness of conical remodeled stent-grafting, the appropriate size, and type of stent-graft for small visceral artery remains to be disclosed.
Figures and Tables
Fig. 1
A proper hepatic artery pseudoaneurysm in a 56-year-old male patient after living-donor liver transplantation.
A. CT angiography on postoperative day 15. On axial CT images, the pseudoaneurysm (arrowhead) is located in the posteroinferior aspect of the hepatic artery proper (arrow). A peri-hepatic hematoma (curved arrow) is seen.
B. On postoperative day 23, celiac arteriography shows faint visualization of the pseudoaneurysm (arrowheads). Aneurysmal change of the ligated right hepatic artery stump (arrow) is also seen.
C. The entire stent-graft is first expanded to 3.33 mm (oversizing by 20% to the distal target vessel) by ballooning up to 15 atm.
D. The deflated balloon is pulled back slightly proximal to the stent-graft, then inflated up to 19 atm, making the diameter 3.95 mm (oversizing by 20%) for the proximal target vessel.
E. Post-deployment celiac arteriography shows the conical remodeled stent-graft and complete exclusion of the pseudoaneurysm.
F. Maximum intensity projection image shows patent stent-graft without any evidence of pseudoaneurysm on follow-up CT angiography 17 months later.
CT = computed tomography
References
1. Marshall MM, Muiesan P, Srinivasan P, Kane PA, Rela M, Heaton ND, et al. Hepatic artery pseudoaneurysms following liver transplantation: incidence, presenting features and management. Clin Radiol. 2001; 56:579–587.

2. Saad WE, Dasgupta N, Lippert AJ, Turba UC, Davies MG, Kumer S, et al. Extrahepatic pseudoaneurysms and ruptures of the hepatic artery in liver transplant recipients: endovascular management and a new iatrogenic etiology. Cardiovasc Intervent Radiol. 2013; 36:118–127.

3. Muraoka N, Uematsu H, Kinoshita K, Takeda T, Morita N, Matsunami H, et al. Covered coronary stent graft in the treatment of hepatic artery pseudoaneurysm after liver transplantation. J Vasc Interv Radiol. 2005; 16:300–302.

4. Elias G, Rastellini C, Nsier H, Nazarey P, Brown M, Pahari M, et al. Successful long-term repair of hepatic artery pseudoaneurysm following liver transplantation with primary stent-grafting. Liver Transpl. 2007; 13:1346–1348.

5. Hamby BA, Ramirez DE, Loss GE, Bazan HA, Smith TA, Bluth E, et al. Endovascular treatment of hepatic artery stenosis after liver transplantation. J Vasc Surg. 2013; 57:1067–1072.

6. Saxon RR, Chervu A, Jones PA, Bajwa TK, Gable DR, Soukas PA, et al. Heparin-bonded, expanded polytetrafluoroethylene-lined stent graft in the treatment of femoropopliteal artery disease: 1-year results of the VIPER (Viabahn Endoprosthesis with Heparin Bioactive Surface in the Treatment of Superficial Femoral Artery Obstructive Disease) trial. J Vasc Interv Radiol. 2013; 24:165–173. quiz 174.

7. Amblard A, Berre HW, Bou-Saïd B, Brunet M. Analysis of type I endoleaks in a stented abdominal aortic aneurysm. Med Eng Phys. 2009; 31:27–33.

8. Lu NN, Huang Q, Wang JF, Wei BJ, Gao K, Zhai RY. Treatment of post-liver transplant hepatic artery pseudoaneurysm with balloon angioplasty after failed stent graft placement. Clin Res Hepatol Gastroenterol. 2012; 36:e109–e113.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download