Abstract
Secretory carcinoma of the breast is an extremely rare, clinically and histologically distinct variant of invasive ductal carcinoma, with an indolent growth pattern and a more favorable prognosis than that of typical ductal carcinoma. Few studies have described its imaging features. Herein, we report on a secretory breast carcinoma with findings from various imaging modalities, especially including the MRI findings, which appears a well-defined complex cystic mass. Awareness of its imaging features using various modalities will be helpful for the differential diagnosis.
초록
분비성 유방암은 매우 드물지만, 임상 그리고 병리학적으로 구분되는 침습성 유관암의 한 종류로, 전형적인 침습성 유관암보다 덜 침습적이고 더 좋은 예후를 가지고 있는 것으로 알려져 있다. 매우 적은 연구들에서만 이 병의 영상 소견에 대해 서술하였다. 이에 저자들은 경계가 좋은 복합 낭성 종괴로 보이는 분비성 유방암의 자기공명영상을 포함한, 다양한 영상들의 소견에 대해 보고하고자 한다. 이 병의 여러 영상 소견을 알고 있는 것은 감별진단에 도움이 될 것이다.
Secretory breast carcinoma (SBC) is an extremely rare but distinct subtype of breast carcinoma, accounting for less than 1% of all infiltrating breast carcinomas (1). A review of available literatures shows that most reports of cases of SBC include histopathological findings (2345). The tumors were characterized by existence of abundant eosinophilic secretions in the intracellular vacuoles and the intercellular spaces (3). The carcinoma is reported to have better prognosis than a typical ductal carcinoma (14). However, the imaging findings of this carcinoma are not well-described. In this report, we describe the case of an 80-year-old woman with SBC. The case report highlights imaging findings on various modalities, especially MRI findings.
An 80-year-old woman patient had noticed a lump in the upper-outer quadrant of the right breast. She presented to the local clinic for examination. At the time, ultrasound (US) examination and core needle biopsy of the palpable mass were conducted. She was diagnosed with secretory carcinoma in her right breast. She visited our hospital for further examination and treatment. There was no family history of relevant malignancy. Upon physical examination, the palpable mass in the breast was found to be painless, firm, and well-circumscribed, with no nipple discharge or inversion.
Mammography showed a 9.5-cm sized, hyperdense, well-defined ovoid mass, without associated suspicious microcalcifications or architectural distortion (Fig. 1A). US of the breast revealed a more than 5-cm sized complex cystic mass with relatively circumscribed margin in the right breast which was over the field of view (FOV) of US (Fig. 1B) and there was a suspicious lymph node with eccentric cortical thickening in the right axilla. MRI examination of the breast revealed a 9-cm sized complex cystic mass in the right breast (Fig. 1C). The solid component of the mass had initial fast and delayed washout enhancement (Fig. 1C). Maximum standardized uptake value of the solid portion of this tumor was 11.4 on PET/CT (Fig. 1D). Microscopic findings demonstrated abundant eosinophilic secretory material in both cells and tubular acini, and tumor cells that were vacuolated with abundant secretory materials (Fig. 1E) (3). In addition, immunohistochemical examination revealed tumor cells that were negative for estrogen receptors and progesterone receptors, positive for human epidermal growth factor receptor 2 (HER2), and weakly positive for Ki67 (10% positive cells). The clinical stage was cT4N1M0 and stage IIIB based on skin edema in the inner portion of the right breast on mammography, US and MRI and a suspicious axillary lymph node on US.
Surgery was planned after completion of chemotherapy; however, the patient died after 8 cycles of chemotherapy, probably due to pneumonia and pulmonary edema. Therefore, there was no surgical pathology.
Secretory carcinoma is a rare breast carcinoma that accounts for less than 1% of all infiltrating breast carcinomas (1). It was first termed “juvenile breast carcinoma” by McDivitt and Stewart in 1966 (6), because they reported seven cases of secretory carcinoma which occurred exclusively in young children with an average of nine years. Subsequently, studies have shown that it affects all age groups, and based on its prominent secretory component, it is classified as secretory carcinoma by Tavassoli and Norris (7). The typical clinical presentation includes a slow-growing, painless, well-circumscribed, mobile, and palpable mass. Axillary lymph node metastasis is uncommon in patients with tumors of smaller than 2-cm size (7). Unlike HER2 type breast carcinoma in our case, secretory carcinomas are generally negative for estrogen and progesterone receptors and usually do not show HER2 gene amplification (triple negative breast carcinoma) (24). Even though most SBC are triple negative breast carcinoma, the prognosis is generally excellent, it may be due to the heterogeneity of histologic types (4).
Previous case reviews showed that mammographic findings of SBC were variable and non-specific, ranging from no abnormal findings or benign-looking nodular density to suspicious malignant lesions with spiculated margins or microcalcifications (8). In our case, mammography showed a well-defined large ovoid mass without suspicious microcalcifications or architectural distortion, as seen in benign lesions, and these findings were not specific for the entity of SBC. Mun et al. (8) reported that US in patients with SBC showed small well-circumscribed or partially microlobulated isoechoic or hypoechoic nodules, indistinguishable from some benign masses, as well as other well-circumscribed malignancies. The lesion is usually homogeneous, but can be heterogeneous. In our case, US revealed a more than 5-cm sized complex cystic mass with relatively circumscribed margin, which are nonspecific findings. The MRI features of SBC are not well known. In our case, MRI examination revealed a 9-cm sized complex cystic mass in the right breast. In the mass, the solid component had initial fast and delayed washout enhancement pattern on dynamic study. Whereas mammography shows the tumor completely but couldn't give information of mass component and US has limitation of FOV for a large mass, MRI could demonstrate the whole mass component and the exact location of solid portion to guide surgical plan.
We have reviewed previously reported cases of SBC in Table 1. SBC can occur in all age groups, but the median age is under the age of sixty and the median tumor size is less than 3-cm. On the radiologic report of SBC, the tumor generally appeared benign looking solid mass on mammography and US (8). However, in our case, the patient was 80-year old woman and had a large mass. In addition, US and MRI demonstrated a complex cystic mass which was different from the previous studies, although mammography showed a well-defined ovoid mass.
SBC is slow-growing and can mimic benign lesions, such as fibroadenoma; and the differential diagnosis includes a wide range of benign or malignant lesions. Based on the imaging characteristics including MRI findings, differential diagnoses include papillary carcinoma, matrix-producing carcinoma, high grade invasive ductal carcinoma with necrosis. In case of papillary carcinoma, it can present as a hypoechoic solid mass or a complex cyst with septations or mural-based papilliform nodularity on US (9). However, it has been reported that secretory carcinoma of the breast could show associated ductectasia or cystic portion with features that mimic intraductal papillary lesions (8). Therefore, it is difficult to differentiate SBC from papillary carcinoma. The matrix-producing carcinoma manifests as a microlobulated, isoechogenic solid mass with cystic components on US. On MRI, T2-weighted images show a relatively well-defined mass with internal high signal intensity necrotic or cystic components and there is early enhancement and a delayed washout in a peripheral rim and nonenhancing internal components (10). If there were osteoid or chondroid calcifications on mammography, they were specific findings of matrix-producing carcinoma than other tumors. High grade invasive ductal carcinomas with necrosis have non specific MRI findings. They were more likely than expected to display microlobulated margins, abrupt interfaces, and posterior enhancement on US. High grade invasive ductal carcinoma can be considered when the mass shows irregular central cystic portion suggesting necrosis rather than cystic lesion with papillary growing solid mass on US and suspicious microcalcifications on mammography.
However, these findings should be interpreted with caution, when SBC grows over than 5 cm, which can show a well-defined complex cystic mass, it is difficult to differentiate malignant from benign lesions. Hence, core needle biopsy of a solid portion is essential for early detection of secretory carcinoma, in order to prevent delayed diagnosis of malignancy.
In conclusion, although SBC is very rare, it could manifest as a well-defined complex cystic mass with a solid component that has initial fast and delayed washout-enhancement on MRI. Awareness of its imaging features using various modalities will be helpful for the differential diagnosis.
Figures and Tables
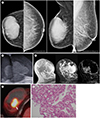 | Fig. 1Secretary breast carcinoma in an 80-year-old woman.A. A hyperdense circumscribed mass without suspicious microcalcifications is seen in the right breast on the craniocaudal and mediolateral oblique views.
B. Ultrasound shows a greater than 5-cm complex cystic mass in the right breast.
C. T1WI demonstrates low signal intensity in the central solid portion (arrow); and intermediate and high signal intensities in the cystic portion of the mass, which is probably due to hemorrhage caused by core needle biopsy. T2WI with fat saturation demonstrates low signal intensity in the central solid portion (arrow); and high signal intensity in the cystic portion of the mass. Subtracted gadolinium enhanced T1WI shows a large thin-rim enhancement with an eccentric enhancing solid portion (arrow).
D. PET/CT image shows a large complex cystic mass with a hypermetabolic solid portion (maximum standardized uptake value 11.4) (arrow).
E. Histologic specimen shows abundant eosinophilic cytoplasm and intracellular and extracellular eosinophilic secretory material (hematoxylin and eosin staining, ×200).
T1WI = T1-weighted image, T2WI = T2-weighted image
|
References
1. Horowitz DP, Sharma CS, Connolly E, Gidea-Addeo D, Deutsch I. Secretory carcinoma of the breast: results from the survival, epidemiology and end results database. Breast. 2012; 21:350–353.

2. Jena M, Shariff S. Cytodiagnosis of secretory carcinoma of the breast: a report on two cases. Diagn Cytopathol. 2010; 38:921–924.

3. Akhtar M, Robinson C, Ali MA, Godwin JT. Secretory carcinoma of the breast in adults. Light and electron microscopic study of three cases with review of the literature. Cancer. 1983; 51:2245–2254.

4. Vasudev P, Onuma K. Secretory breast carcinoma: unique, triple-negative carcinoma with a favorable prognosis and characteristic molecular expression. Arch Pathol Lab Med. 2011; 135:1606–1610.

5. Li D, Xiao X, Yang W, Shui R, Tu X, Lu H, et al. Secretory breast carcinoma: a clinicopathological and immunophenotypic study of 15 cases with a review of the literature. Mod Pathol. 2012; 25:567–575.

8. Mun SH, Ko EY, Han BK, Shin JH, Kim SJ, Cho EY. Secretory carcinoma of the breast: sonographic features. J Ultrasound Med. 2008; 27:947–954.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download