Abstract
Primary renal neuroendocrine tumor (NET) is an extremely rare disease with fewer than 100 reported cases to date. Among them, only three involved the renal pelvis, to our knowledge. Here, we report another rare case of primary NET in the renal pelvis of a 33-year-old man. Initial computed tomography (CT) scanning of the abdomen and pelvis revealed a necrotic mass with peritumoral infiltration at the left renal pelvis and ureteropelvic junction causing urinary tract obstruction. A follow-up CT scan revealed an intratumoral hemorrhage. The patient then underwent nephrectomy. The results of a subsequent histopathological examination were consistent with a well-differentiated NET. No lymph nodes or paraganglia were found within the tumor, and further imaging revealed no other primary or metastatic lesions. Therefore, the patient was diagnosed with primary NET in the renal pelvis. We discuss this rare case and briefly review the current NET literature.
Neuroendocrine tumors (NETs) consist of a heterogeneous group of malignancies, defined as epithelial neoplasms with predominant neuroendocrine differentiation. They can arise in most organs of the body but are most commonly found in the gastrointestinal tract and lungs. Primary renal NETs are extremely rare, and the number of reported cases in the literature was fewer than 100 since its first report described by Lamb (2015) and Resnick et al. (1966) (12), NET in the renal pelvis is even rarer, representing only three of the aforementioned patients but with no specific mention of radiologic findings in these prior case reports (345). Here, we present a case of primary NET arising from the renal pelvis with hydronephrosis that mimics a complicated cyst.
A 33-year-old otherwise healthy man was admitted to our hospital with left flank pain and diaphoresis. He had stable vital signs, without symptoms or signs suggestive of a systemic inflammatory response or carcinoid syndrome. All laboratory test results including urinalysis were normal, except for an elevated C-reactive protein level (5.52; normal range, 0.0–0.3 mg/dL). A computed tomography (CT) scan of the abdomen and pelvis revealed a thick-walled ovoid-shaped hypodense mass measuring 4.2 × 3.9 × 5.2 cm abutting the left renal pelvis and ureteropelvic junction (Fig. 1). The tumor showed peritumoral infiltration involving both the peripelvic and renal sinus fat and was located near the lower pole of the left renal parenchyma. However, no gross invasion to the renal parenchyma could be clearly demonstrated. The center of the tumor was found to be slightly hyperdense compared to the dilated calyx, measuring approximately + 30 HU on a precontrast CT. Peripheral mild enhancement was suspected (an attenuation difference of approximately 30–45 HU was noted); however, the attenuation difference was difficult to measure because the wall of the cyst was not sufficiently thick. The left kidney showed diffuse hydronephrosis and parenchymal atrophic changes that were indicative of a chronic obstruction. Focal calcification was also observed at the peripheral portion of the tumor. A tumor thrombus was not observed in the renal vein or inferior vena cava, and no metastatic lesions were identified.
A follow-up CT scan after 3 days revealed that the tumor size had increased with high attenuation at the central portion of the mass that seemed to be a result of hemorrhage within the interval. The first differential diagnosis was therefore a hemorrhagic cyst with an adjacent peritumoral infiltration. However, due to the findings regarding a chronic obstruction, including its size, a hemorrhagic tumor was also included in the differential diagnosis.
A CT-guided biopsy was initially performed to avoid left nephrectomy, although its usefulness was expected to be limited due to the presence of a predominantly hemorrhagic tumor with scantly enhanced solid portions. The resulting histopathological diagnosis was a nonneoplastic cystic lesion with foamy histiocyte aggregates. Given that the left kidney in this patient was almost nonfunctional and the targeting accuracy of the biopsy in cases of necrosis-dominant lesions can be unreliable, radical nephroureterectomy and mass resection were determined as the appropriate approach for both treatment and accurate diagnosis. After a 5-day postoperative course with no adverse events, the patient was discharged from the hospital.
Upon gross examination, the resected mass was 4.5 × 3.5 × 3.2 cm in size and had a grayish yellow color (Fig. 1E). This mass had extended to the renal sinus fat tissue, without parenchymal compromise of the left kidney. Upon sectioning, the mass contained brownish necrotic material. Further histopathology revealed oval and round tumor cells arranged in a trabecular pattern with 3 mitoses/10 high-power fields and a Ki-67 proliferation rate of 11.43%. The tumor cells showed diffuse immunoreactivity for synaptophysin and CD56 and a focal expression of chromogranin A, but did not express PAX-8. The aforementioned histopathological characteristics supported our diagnosis of a well-differentiated NET. Additional chest CT and bone scans did not detect any metastatic disease.
NETs represent a broad spectrum of diseases including well-differentiated lesions as in our current case and both well- and poorly differentiated neuroendocrine carcinoma (6). Well-differentiated NETs are typically slow-growing tumors occurring at an age below 50 years. Majority of the affected patients also usually present with nonspecific symptoms, such as pain, obstruction, or hematuria. In a minority of NET cases, patients may have symptoms related to hormone production, such as skin flushing or diarrhea, which is known as the carcinoid syndrome. NET in the renal pelvis is an extremely rare form of this neoplasm, and only three cases were reported to our knowledge (345). The primary renal NET can have coexisting renal anomalies, such as horseshoe kidney (22.3%) and teratomas (11.8%) (1). Among the cases not associated with a horseshoe kidney, the majority (77.3%) were located in the renal parenchymas and 4.5% were located in the renal pelvis (1). The small number of reported NETs arising from the renal pelvis has not been associated with renal anomalies (5). The histogenesis of NET lesions occurring in the kidney or renal pelvis is uncertain, but the urothelial epithelium at the multipotent reserve cell level has been suggested as its origin. These tumors may also possibly develop from the neuroendocrine cells that have been identified in the normal mucosa of the renal pelvis and ureter (5).
No specific radiologic features have yet been established to distinguish renal cell carcinomas or other renal tumors from NET lesions. According to a previous review of case series published on these cancers to date, renal NETs present as a well-defined mass with marked (18%) or mild (14%) enhancement, with accompanying calcifications in one third of cases (1). Unlike the pancreatic NETs that are known to be hyperattenuated masses in arterial and venous phases due to a rich capillary network (7), renal NETs may show a variable degree of enhancement, from mild to marked, making the correct preoperative diagnosis difficult. NETs tend to appear as heterogeneous entities due to cystic changes, calcification, and necrosis as its size increases (7). A predominantly cystic appearance, typically involving a central unilocular region of cystic degeneration, is observed in approximately 5–10% of pancreatic NET lesions (7). According to one previous report (8), cystic NETs are more symptomatic than solid tumors, which is consistent with our observations in the present study case. Recent and intermittent intra-tumor bleeding may have contributed to the onset of symptoms in our patient.
The revised World Health Organization (WHO) classification in 2017 divided gastroenteropancreatic NETs into NET G1, NET G2, and NET G3 categories based on mitotic activity, Ki67 immunostaining, and tumor morphology (6). Among them, NET G3 is considered poorly differentiated and referred to as neuroendocrine carcinoma. Renal NET does not belong to a specific classification system; our present case could be classified as G2 in accordance with these new guidelines. A study by Takumi et al. (9), based on the 2000 WHO classification, reported that tumor contrast enhancement during the portal venous phase was significantly higher in G1 than in G2 disease (p < 0.001) and that a non-hyperattenuating NET was suggestive of a G2 category (p = 0.016). Although this study did not exclusively evaluate cystic tumors, it indicated that the degree of contrast enhancement could potentially be used to estimate the pathologic grade. Moreover, this could also explain the non-hyperattenuation in our current case, which is a G2 NET.
In the pathologic diagnosis of NETs, immunohistochemistry plays a pivotal role in firstly identifying the neuroendocrine differentiation. Among the several immunohistochemical markers described to date, the concurrent use of chromogranin A and synaptophysin that are most widely used and known to be reliable is recommended to accurately confirm the neuroendocrine differentiation in a suspected well-differentiated NET (10). In our case, the mass expressed both chromogranin A and synaptophysin expression as well as CD56, a less specific marker for neuroendocrine differentiation. Meanwhile, PAX-8 is a renal-lineage transcription factor and is used as a diagnostic marker for renal cell carcinoma. The absence of PAX-8 expression in our case supports that the mass was not originated from the renal epithelial cells.
In summary, we present a case of an NET presenting as a hemorrhagic and necrotic mass with an equivocally enhanced thick wall in the renal pelvis that was misdiagnosed as a hemorrhagic cyst. Due to the rarity of this tumor, making a correct diagnosis with primary renal NET preoperatively may be difficult. However, NET can be considered in cases presenting with a hemorrhagic mass outpouching from the renal pelvis, especially in younger patients.
Figures and Tables
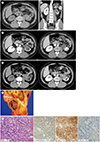
Fig. 1
A 33-year-old man with neuroendocrine tumor of the renal pelvis presenting with left flank pain.
A. Precontrast CT scan reveals a hypodense mass with peripheral calcification (arrow) abutting the left renal pelvis. The center of the tumor is slightly hyperdense with a value of approximately +30 HU.
B. A coronal reformatted post-contrast CT scan shows diffuse hydronephrosis and parenchymal atrophic changes in the left kidney, which are indicative of a chronic obstruction.
C. On the contrast-enhanced CT scan, a thick-walled mass shows prominent peritumoral infiltration (arrow-heads) and suspicious peripheral mild enhancement (attenuation difference, approximately 30–45 HU).
D. The follow-up CT scan shows high attenuation at the central portion of the mass (arrows) on precontrast (left panel) and enhanced (right panel) images.
CT = computed tomography, HU = Hounsfield unit
E. On gross examination, a 4.5 × 3.5 × 3.2-cm well-demarcated and grayish yellow-colored mass is found to extend to the renal sinus fat tissue but is separated from the renal parenchyma (arrows).
F. The mass is composed of oval and round tumor cells arranged in a trabecular pattern on microscopic examination. Immunohistochemical staining demonstrated that neuroendocrine tumor cells have diffuse immunoreactivity to synaptophysin and CD56, without expression of PAX-8.
References
1. Lamb L, Shaban W. Primary renal carcinoid tumor: a radiologic review. Radiol Case Rep. 2015; 9:923.

2. Resnick ME, Unterberger H, McLoughlin PT. Renal carcinoid producing the carcinoid syndrome. Med Times. 1966; 94:895–896.
3. Rudrick B, Nguyen GK, Lakey WH. Carcinoid tumor of the renal pelvis: report of a case with positive urine cytology. Diagn Cytopathol. 1995; 12:360–363.

4. Ji X, Li W. Primary carcinoid of the renal pelvis. J Environ Pathol Toxicol Oncol. 1994; 13:269–271.
5. Kuroda N, Katto K, Tamura M, Shiotsu T, Hes O, Michal M, et al. Carcinoid tumor of the renal pelvis: consideration on the histogenesis. Pathol Int. 2008; 58:51–54.

6. Kruljac I, Pape U. The classification of neuroendocrine neoplasms: “neuroendocrine carcinomas” revisited—a 2017 update and future perspectives. Endocr Oncol Metab. 2017; 3:37–42.
7. Lewis RB, Lattin GE Jr, Paal E. Pancreatic endocrine tumors: radiologic-clinicopathologic correlation. Radiographics. 2010; 30:1445–1464.

8. Bordeianou L, Vagefi PA, Sahani D, Deshpande V, Rakhlin E, Warshaw AL, et al. Cystic pancreatic endocrine neoplasms: a distinct tumor type? J Am Coll Surg. 2008; 206:1154–1158.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download