Abstract
Papillary meningioma is rare meningeal tumors and is associated with aggressive clinical behavior as compared with other meningiomas. We report a case of papillary meningioma in a 50-year-old woman presented with complaints of headache, nausea and vomiting. MRI revealed a very macrolobulated heterogeneously enhancing solid mass at the right frontal convexity with focal prominent inward infiltrating portion and surrounding moderate brain edema. The localization of the lesion as intra-axial or extra-axial in origin was difficult. Demonstrated diffusion restriction and high relative cerebral blood volume value were similar to conventional meningioma, but hypervascular inward infiltrating portion could be seen in papillary meningioma. The histopathology examination of the resected tissues revealed papillary meningioma with an increased cellularity and high nuclear/cytoplasm ratio. She underwent radical excision of the tumor, followed radiotherapy and tumor recurrence occurred at 12 months later.
Meningiomas account for 24–30% of primary intracranial tumors diagnosed in the USA (1). The majority of meningiomas are benign, slow growing tumors with good prognoses. However, papillary meningioma (PM) is an aggressive histological variant of meningioma, accounting for 1.0–2.5% of all intracranial meningiomas diagnosed (1). According to the 2016 revision of the World Health Organization (WHO) tumor classification system (2), PM is pathologically identified as Grade III in cases where a perivascular or pseudopapillary pattern is present (3). At the time of writing, only a limited number of PM cases have been reported. We report a case of PM in a 50-year-old woman and discuss its imaging findings.
A 50-year-old woman presented with a history of headache, nausea and vomiting lasting for 2 weeks. Neurological examination did not reveal any focal neurological deficit, and her past medical history was otherwise unremarkable.
The contrasted brain MRI showed a macrolobulated margin, focal prominent inward infiltrating portion, and moderate surrounding brain edema. The mass was located at the right perisylvian inferior frontal convexity, but localization of lesion origin as intra-axial or extraaxial was difficult. The mass, which was predominantly solid with several intratumoral microcysts, measured 5.1 × 4.7 × 5.2 cm and showed mass effect compressing the right lateral ventricle. Midline shifting to the left side was also seen. Additionally, the mass showed both encasement of the right frontal middle cerebral artery branches, and peripheral engorged veins. The lesion showed heterogeneous intermediate signal intensity on T1 and T2 weighted images, and post-contrast heterogeneous enhancement (Fig. 1A). A CT scan revealed a macrolobulated, and heterogeneous enhancing mass at the right perisylvian inferior frontal convexity, with surrounding brain edema (Fig. 1B). The lesion demonstrated restricted diffusion (Fig. 1C), and high relative cerebral blood volume (rCBV) with an inward high rCBV portion (Fig. 1D).
A right craniotomy and tumor debulking procedure was performed. Intraoperatively, the tumor was very firm and hard, and the tumor margin showed severe adhesion with brain tissue and invasion of brain parenchyma.
The histopathological examination of the resected tissues revealed an increased cellularity, small cells with high nuclear-cytoplasmic ratio, and spontaneous or geographic necrosis. A perivascular pseudopapillary pattern was also seen in the resected tissue (Fig. 1E). The cells were mitotically active (mitosis 7–9/10 HPF), and prominent nucleoli were not observed. Immunohistochemistry revealed tumor cells positive for epithelial membrane antigen and vimentin.
The patient was later referred to an oncology unit for radiotherapy. Twelve months later the tumor recurred, and 21 months later the tumor worsened and leptomeningeal metastasis developed (Fig. 1F).
PM is a malignant variant of meningioma first described in 1938 by Cushing and Eisenhardt (4). They reported a papillary pattern in a meningioma showing intracerebral recurrence and pulmonary metastasis. PM is frequently seen in the supratentorial compartment, though rare locations such as the posterior fossa, jugular foramen, and oculomotor nerve have been described (5).
Patients with PMs usually manifest symptoms caused by intracranial hypertension, such as severe headache, vomiting, and blurred vision, which are notably alleviated after resection of the tumor (5). PMs are more commonly seen in males and tend to occur in younger patients (6). In contrast with typical benign meningioma (WHO Grade I), our patient's MR scan showed a macrolobulated margin, which was attributed to a highly heterogeneous distribution of proliferating cells in the PM tumor, and resulted in an imbalanced cell density, and disproportionate intratumoral pressure. In our study, the tumor showed invasion of brain parenchyma, which indicates the absence of physiological barriers between the tumor and adjacent brain parenchyma. The invaded portion showed increased cerebral blood volume value and demonstrated diffusion restriction. Intratumoral microcystic change and heterogenous enhancement were also seen in this case, potentially due to microcystic degeneration, ischemic necrosis, or hemorrhage within the tumor. In previous reports, similar to our findings, high grade meningiomas usually have unclear tumor margins, heterogeneous gadolinium enhancement, and a larger scale of peritumoral brain edema (7). According to Wang et al. (5), PM shows irregular tumor margins, heterogenous enhancement, and severe peritumoral brain edema in the absence of a peritumoral rim. The presence of a tumor cyst is an exceptional finding in meningiomas, but it has been frequently reported in PM (8) in addition to our case. Yu et al. (9), who previously reported the largest and most recent series of PMs, mentioned that the MRI features of PM are unclear tumor-brain interface, and internal heterogeneity, including cyst formation, irregular enhancement, signal voids of vessels, and marked peritumoral edema. Our study showed comparable MRI features.
Due to the rarity of PM, no clear consensus exists regarding the appropriate management of the disease. However, the combination of aggressive surgical resection and postoperative radiotherapy has emerged as a standard treatment strategy for PM (5). PM is generally associated with a high mortality rate. In fact, Wang et al. (5) reported that the 3-year mortality rate for PM was 50–63.6%. In addition, local recurrence of PM has been reported in 56.5% of cases, which is significantly higher than the 10-20% recurrence rate reported for conventional meningiomas (5). The lung is the most common site of extracranial metastasis (5/10, 50%) (10).
PMs need to be differentiated from other intracranial tumors because they are malignant and have the potential for extracranial metastasis. Their timely detection could prevent local and distant metastasis, and the mortality or morbidity associated with it. The imaging findings mentioned above may serve to aide diagnosis for PM.
Figures and Tables
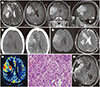 | Fig. 1A 50-year-old female with papillary meningioma in the right frontal region.
A. MR images show a macrolobulated mass in right frontal lobe with a focal prominent inward infiltrating portion. The axial T2-weighted image shows an irregular mass with intermediate signal intensity at the right perisylvian inferior frontal convexity, measuring 5.1 × 4.7 × 5.2 cm. The lesion extends to deep white matter with mass effect, compressing the right lateral ventricle. It is predominantly solid with several intratumoral microcystic components with moderate surrounding brain edema, and peripheral engorged veins. Post-contrast T1-weighted axial, coronal, and sagittal images show heterogeneous enhancement of the tumor.
B. The pre-contrast axial CT scan shows a large, irregular heterogeneous increased attenuated mass at the right perisylvian inferior frontal convexity. Note the mass shows moderate surrounding brain edema. Post-contrast axial CT of the lesion shows an inhomogeneously enhancing soft tissue mass.
C. DWI and the ADC show diffusion restriction of the tumor.
D.Perfusion MRI shows an increased relative cerebral blood volume value within the mass, especially in inward infiltrating portion.
E.On histopathological examination, the tumor shows increased cellularity, a perivascular pseudopapillary pattern with rhabdoid features, and pleomorphism (haematoxylin and eosin stain, × 400).
F. After 21 months, an axial gadolinium-enhanced follow-up MR image shows a recurrence of the tumor along the surgical bed of the right perisylvian frontotemporal area.
ADC = apparent diffusion coeffcient, DWI = diffusion-weighted imaging
|
References
1. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 14:97–109.

2. Johnson DR, Guerin JB, Giannini C, Morris JM, Eckel LJ, Kaufmann TJ. 2016 updates to the WHO brain tumor classification system: what the radiologist needs to know. Radiographics. 2017; 37:2164–2180.
3. Buccoliero AM, Caldarella A, Taddei A, Di Lorenzo N, Gallina P, Mennonna P, et al. Atypical, aplastic, and unusual meningiomas. Morphology and incidence in 300 consecutive cases. Pathologica. 2003; 95:83–88.
4. Cushing H, Eisenhardt L. Meningiomas: their classification, regional behaviour, life history, and surgical end results. Springfield: Charles C. Thomas;1938. p. 785.
5. Wang DJ, Zheng MZ, Gong Y, Xie Q, Wang Y, Cheng HX, et al. Papillary meningioma: clinical and histopathological observations. Int J Clin Exp Pathol. 2013; 6:878–888.
6. Kros JM, Cella F, Bakker SL, Geuze DP, Egeler RM. Papillary meningioma with pleural metastasis: case report and literature review. Acta Neurol Scand. 2000; 102:200–202.

7. Kawahara Y, Nakada M, Hayashi Y, Kai Y, Hayashi Y, Uchiyama N, et al. Prediction of high-grade meningioma by preoperative MRI assessment. J Neurooncol. 2012; 108:147–152.

8. Vaquero J, Zurita M, Coca S. Cystic papillary meningioma. Neuropathol Appl Neurobiol. 2002; 28:418–421.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download