This article has been
cited by other articles in ScienceCentral.
Abstract
Thiamine (vitamin B1) is a water-soluble vitamin that is not endogenously synthesized in humans. It is absorbed by the small intestine, where it is activated. Its active form acts as a coenzyme in many energy pathways. We report a rare case of thiamine deficiency in a 3.5-year old boy with short bowel syndrome secondary to extensive bowel resection due to necrotizing enterocolitis during his neonatal age. The patient was parenteral nutrition-dependent since birth and had suffered from recurrent central catheter-related bloodstream infections. He developed confusion with disorientation and unsteady gait as well as profound strabismus due to bilateral paresis of the abductor muscle. Based on these and a very low thiamine level he was diagnosed and treated for Wernicke encephalopathy due to incomplete thiamine acquisition despite adequate administration. He fully recovered after thiamine administration. After 1999 eight more cases have been reported in the PubMed mostly of iatrogenic origin.
Go to :

Keywords: Wernicke encephalopathy, Total parenteral nutrition, Thiamine deficiency, Short bowel syndrome
INTRODUCTION
Thiamine or vitamin B
1 is a water-soluble vitamin that is not endogenously synthesized in humans and therefore must be provided through food. It is absorbed by the small intestinal epithelial cells, mainly in the jejunum, where it is phosphorylated into its active form, pyrophosphate thiamine, which acts as a coenzyme in many energy pathways [
12].
Unlike other vitamins of the B complex family, such as B12, thiamine is not stored in tissues in large amounts. A healthy diet provides sufficient thiamine stores for a few weeks under normal circumstances. Foods rich in thiamine are liver, meat, milk, cereals, nuts, and legumes. Diarrhea can largely affect the amount of thiamine absorbed and stored, leading to shortage rather quickly.
Traditionally, in the pediatric population, thiamine deficiency has been attributed to poor socioeconomic status and low availability of thiamine in Asian rice-based diets. A classic syndrome developing in such settings is beri-beri [
3]. However, food fortified worldwide with thiamine has significantly decreased the number of these cases, and deficiency is now noted in settings of acute or chronic disease, such as cancer and gastrointestinal disease. In children, a sequel to such a deficiency is non-alcoholic Wernicke encephalopathy. Typical clinical findings described are ophthalmoplegia, ataxia, and deterioration of conscience, although these findings are not always present in the pediatric population [
3]. Brain magnetic resonance imaging (MRI) reveals specific pathognomonic findings, which usually make or confirm the diagnosis [
4].
For children critically ill because of malignancies or gastrointestinal diseases, adequate nutrition and caloric intake should be provided through parenteral nutrition (PN). Total parenteral nutrition (TPN) with inadequate vitamin supplementation remains an important predisposing factor for thiamine deficiency [
5].
Home PN is often administered via industrially ready three-chamber plastic bags. Adjustments to reach caloric goals per age and weight are made by removing certain quantities per bag compartment. Trace elements and vitamins are not included and are added separately. Thiamine is included in a multivitamin product along with other water-soluble vitamins.
In this report, we describe a case of a 3.5-year-old boy with short bowel syndrome presenting Wernicke encephalopathy due to vitamin B1 deficiency; we also review the literature. Our patient differed from the previously presented cases because he was being adequately treated with thiamine.
Go to :

CASE REPORT
The 3.5-year-old boy with PN dependence was referred to our institution at 2.5 years of age because of severe malnutrition and recurrent central catheter-related bloodstream infections.
He was born prematurely, at the gestational age of 30 weeks. At day 5 he presented with necrotizing enterocolitis, and a very large portion of his bowel was resected because of extensive ischemic and necrotic lesions. The remaining bowel included 10 cm of jejunum past the ligament of Treitz, surgically lengthened, the ascending and traverse colon and sigmoid. TPN was continued throughout his second year of life, with all attempts to wean him off and increase oral intake failing. His caregivers were trained in how to prepare and administer the PN at home. During his first 2.5 years of life, multiple tunneled central venous catheters were replaced because of accidental removal by the patient or recurrent bloodstream infections and colonization of central catheters with pathogens, which further complicated and increased morbidity and days of hospitalization.
Upon referral to our department at the age of 2.5 years old, the child was on partial PN and oral intake of food. His weight was 10 kg and his height was 83 cm (body mass index [BMI]: 14.5 kg/m2, <5th percentile). Fine motor skills, language, and speech development were all delayed. Overall, his clinical exam was unremarkable aside from the anticipated signs of malnutrition.
PN was continued with addition of lipid- and water-soluble (Soluvit N®; Fresenius Kabi AB, Uppsala, Sweden, including 3.1 mg of thiamine) vitamins and trace elements, and with thorough education of his caregivers on how to prevent central catheter-related infections while at home, along with monthly sessions of ethanol lock therapy aimed at reducing morbidity from infections. Bacterial overgrowth, a common short bowel syndrome complication, was treated with cyclic administration of rifaximin and probiotics. Furthermore, cholestyramine was administered daily to reduce malabsorption and high stool- volume output. No cholestasis or liver disease occurred.
After a year of close follow-up, significant clinical improvement was achieved: weight, 13 kg (weight gain, 3 kg); height, 93 cm; BMI, 15 kg/m2 (25th percentile) no catheter-related infections; minimal stool volume; and decrease of PN volume to 720 mL. The PN provided 66 kcal/kg/day (total, 140 kcal/kg/day), 2.1 g/kg protein, and 2.1 g/kg lipids. Fat-soluble vitamins added to his home PN included retinol (0.69 mg), ergocalciferol (0.01 mg), alpha-tocopherol (6.4 g), and phytonadione (0.2 mg) daily. Water-soluble vitamins were also added daily, including all of the vitamin B complex and ascorbic acid. Finally, trace elements contained in a separate ampoule were added daily; these included 2.5 mg zinc, 0.2 mg copper, 0.1 mg manganese, 0.2 mg selenium, 0.57 mg fluorine, and 0.01 mg iodine. Oral feedings included two servings of commercially fortified fruit sauce and two servings of meat and pasta.
In this clinical setting, our patient presented with acute bilateral onset of strabismus. Preceding this symptom, the caregivers mentioned a 10-day history of decreased appetite, two or three occasions of vomiting and loose stools, low-grade fever, and moments of decreased consciousness. A family history of vomiting and low-grade fever was reported. Upon examination, our patient seemed confused and disoriented, with a profound strabismus. Neurological exam was normal apart from an unsteady gait. His vital signs were within normal range, and he was afebrile. An ophthalmology exam and fundoscopy revealed bilateral paresis of the abductor muscle and no papilledema.
Initial laboratory results showed no electrolyte imbalances (Ca, 9.2 mg/dL; P, 3.8 mg/dL; K, 3.6 mmol/L; Na, 136 mmol/L; Mg, 2.6 mg/dL); normal levels of zinc, 142 μg/dL (reference range, 70–150 μg/dL); no blood gas disturbances (pH, 7.39; HCO
3−, 19 mmol/L; pCO
2, 32 mmHg); normal renal function (blood urea nitrogen, 58 mg/dL; creatinine, 0.54 mg/dL), and normal liver function tests (aspartate transaminase, 34 U/L; alanine transaminase, 19 U/L; glucose, 138 mg/dL; total protein, 6.1 g/dL; albumin, 4.0 g/dL; aluminium phosphide, 294 U/L; total bilirubin, 0.24 mg/dL; γ-glutamyltransferase, 25 U/L; prothrombin time, 13 seconds; international normalized ratio, 1.1). Brain MRI showed bilateral symmetric hyper-intense signals in the periventricular grey matter, in the periaqueductal area, and in both medial thalami (
Fig. 1A). These findings suggested Wernicke encephalopathy, and our patient was treated with intramuscular administration of thiamine. Prior to starting the treatment, serum thiamine was measured, and a lumbar puncture was performed to exclude other infectious causes of encephalopathy. Results from cerebrospinal fluid analysis (culture, biochemical) were negative for infection. Also, an electroencephalogram showed low frequency waves for his age. Blood levels of ethanol prior to and after performing a lock-therapy session were measured, and both were too low to detect. Blood levels of retinol were 0.28 mg/L (reference range, 0.2–0.43 mg/L), of alpha-tocoferol 12.4 mg/L (reference range, 5.5–9 mg/L), of 25-hydroxy vitamin D 11.3 ng/mL, riboflavin 10.7 μg/L (reference range, 3–15 μg/L), B
12 630 pg/mL (reference range, 211–911 pg/mL), and folic acid 6.8 ng/mL (reference range, >5.38 ng/mL).
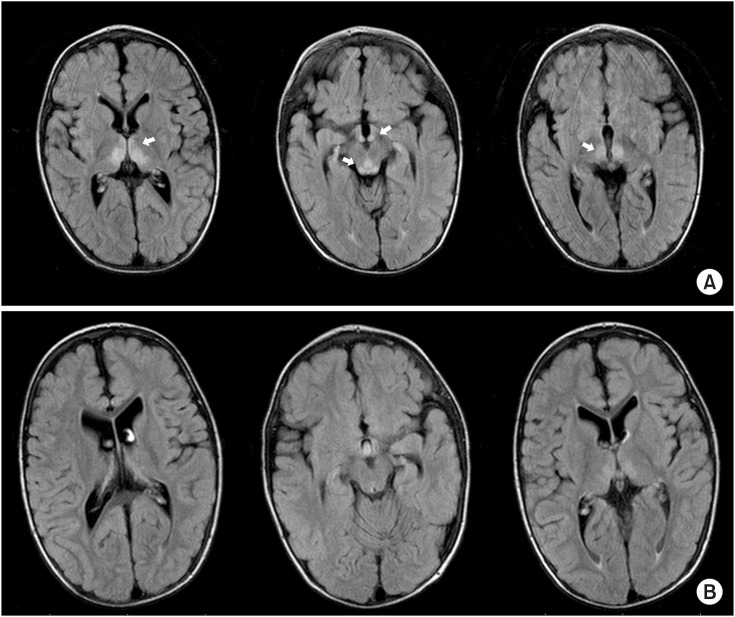 | Fig. 1 (A) Brain magnetic resonance imaging (MRI) findings prior to intramuscular thiamine supplementation therapy: bilateral symmetric hyper-intense signals in the periventricular grey matter, in the periaqueductal area, and both medial thalami (arrows). (B) Brain MRI findings reversed 10 days after therapy.
|
Thiamine was administered intramuscularly (25 mg/day) as part of a multivitamin complex that included vitamins B
1, B
6 and B
12. Improvements in the patient's symptoms were noted in less than 48 hours, and the strabismus completely diminished after three days of supplementation. MRI findings were also reversed 10 days after treatment (
Fig. 1B). Levels of plasma thiamine prior to treatment and 17 days later were 10 and 55 μg/L, respectively (normal values, 28–85 μg/L). Intramuscular thiamine was administered for a total of 17 days.
Go to :

DISCUSSION
The patient reported had been receiving PN for short bowel syndrome secondary to necrotizing enterocolitis during his neonatal period. In comparison to previously reported cases, our patient presented with Wernicke encephalopathy even though he was on daily intravenous vitamin supplementation, including thiamine. Diagnosis was based on clinical findings (ophthalmoplegia and neurocognitive impairment) and medical history, confirmed by brain MRI findings as well as by low plasma levels of thiamine. These were further supported by rapid improvement and complete resolution of symptoms and MRI findings after vitamin B1 administration.
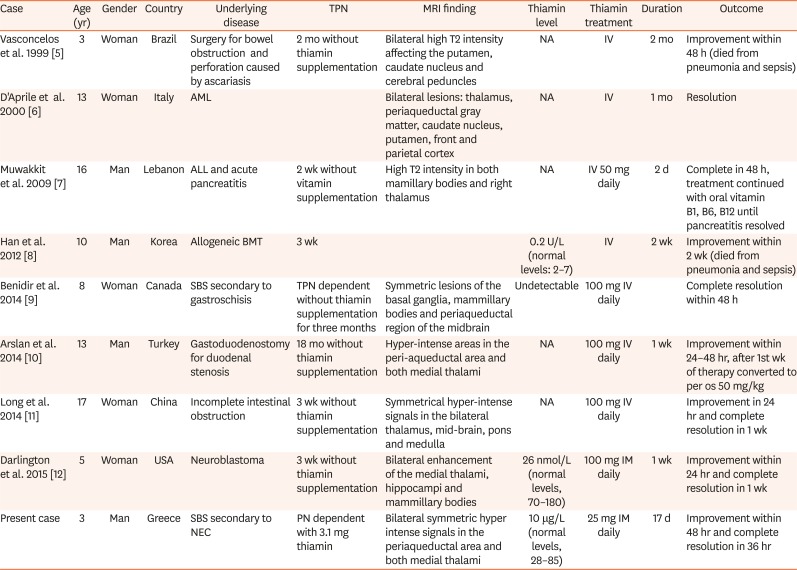
This unusual setting prompted us to review the literature on PubMed using the keywords “total parenteral nutrition”, “Wernicke encephalopathy”, and “children”. We included all cases after 1999 of children below 18 years of age who developed Wernicke encephalopathy while receiving TPN. We found eight cases that met our criteria (
Table 1) [
56789101112].
Table 1
Demographic and clinical features of pediatric patients on total parenteral nutrition with Wernicke's encephalopathy
|
Case |
Age (yr) |
Gender |
Country |
Underlying disease |
TPN |
MRI finding |
Thiamin level |
Thiamin treatment |
Duration |
Outcome |
|
Vasconcelos et al. 1999 [5] |
3 |
Woman |
Brazil |
Surgery for bowel obstruction and perforation caused by ascariasis |
2 mo without thiamin supplementation |
Bilateral high T2 intensity affecting the putamen, caudate nucleus and cerebral peduncles |
NA |
IV |
2 mo |
Improvement within 48 h (died from pneumonia and sepsis) |
|
D'Aprile et al. 2000 [6] |
13 |
Woman |
Italy |
AML |
|
Bilateral lesions: thalamus, periaqueductal gray matter, caudate nucleus, putamen, front and parietal cortex |
NA |
IV |
1 mo |
Resolution |
|
Muwakkit et al. 2009 [7] |
16 |
Man |
Lebanon |
ALL and acute pancreatitis |
2 wk without vitamin supplementation |
High T2 intensity in both mamillary bodies and right thalamus |
NA |
IV 50 mg daily |
2 d |
Complete in 48 h, treatment continued with oral vitamin B1, B6, B12 until pancreatitis resolved |
|
Han et al. 2012 [8] |
10 |
Man |
Korea |
Allogeneic BMT |
3 wk |
|
0.2 U/L (normal levels: 2–7) |
IV |
2 wk |
Improvement within 2 wk (died from pneumonia and sepsis) |
|
Benidir et al. 2014 [9] |
8 |
Woman |
Canada |
SBS secondary to gastroschisis |
TPN dependent without thiamin supplementation for three months |
Symmetric lesions of the basal ganglia, mammillary bodies and periaqueductal region of the midbrain |
Undetectable |
100 mg IV daily |
|
Complete resolution within 48 h |
|
Arslan et al. 2014 [10] |
13 |
Man |
Turkey |
Gastoduodenostomy for duodenal stenosis |
18 mo without thiamin supplementation |
Hyper-intense areas in the peri-aqueductal area and both medial thalami |
NA |
100 mg IV daily |
1 wk |
Improvement within 24–48 hr, after 1st wk of therapy converted to per os 50 mg/kg |
|
Long et al. 2014 [11] |
17 |
Woman |
China |
Incomplete intestinal obstruction |
3 wk without thiamin supplementation |
Symmetrical hyper-intense signals in the bilateral thalamus, mid-brain, pons and medulla |
NA |
100 mg IV daily |
|
Improvement in 24 hr and complete resolution in 1 wk |
|
Darlington et al. 2015 [12] |
5 |
Woman |
USA |
Neuroblastoma |
3 wk without thiamin supplementation |
Bilateral enhancement of the medial thalami, hippocampi and mammillary bodies |
26 nmol/L (normal levels, 70–180) |
100 mg IM daily |
1 wk |
Improvement within 24 hr and complete resolution in 1 wk |
|
Present case |
3 |
Man |
Greece |
SBS secondary to NEC |
PN dependent with 3.1 mg thiamin |
Bilateral symmetric hyper intense signals in the periaqueductal area and both medial thalami |
10 μg/L (normal levels, 28–85) |
25 mg IM daily |
17 d |
Improvement within 48 hr and complete resolution in 36 hr |

In 2009, Francini-Pesenti et al. [
13] reported the recurrence of Wernicke encephalopathy in a case series of adult patients receiving TPN and concluded that vitamin supplementation was not routinely provided. In 1999, Vasconcelos et al. [
5] presented the first case series of pediatric patients with Wernicke encephalopathy. They concluded that children receiving PN comprised the second-largest risk group for developing Wernicke encephalopathy after patients with malignancies. They attributed this finding mainly to a shortage of intravenous multivitamins for PN. Our review of pediatric patients after 1999 showed that all patients who were receiving TPN and ultimately developed Wernicke encephalopathy were not routinely receiving vitamin supplementation. Out of the eight cases, one was due to caregivers' non-compliance [
9] and one to discontinuation after a presumed hypersensitivity reaction [
12]. Therefore, despite no shortage in parenteral vitamin supplementation, iatrogenic non-alcoholic Wernicke encephalopathy remains a problem, which must be addressed with increasing awareness.
The reported patient was regularly receiving five times the recommended daily allowance of thiamine parenterally before he developed Wernicke encephalopathy. A dose of 3.1 mg was added daily to his PN, whereas the recommended daily allowance for his age was 0.6 mg [
2].
Since he was receiving adequate thiamine, several possible causes of thiamine insufficiency were considered, with parental non-compliance being the most likely explanation. First, even though our patient's general condition had improved (increase in BMI, significant improvement in signs of malabsorption, no diarrhea or metabolic acidosis), the recent history of a virus-like illness in the family that also affected our patient (low-grade fever, anorexia, two episodes of vomiting) could have caused a shortage of thiamine because of the excessive requirements [
3].
Second, the method of administration of the PN at home was reviewed with the caregivers to rule out the possibility of degradation of the water-soluble vitamins (including thiamine) because of photosensitivity [
14]. Mixing the water-soluble vitamin preparation and administering it within the lipid-containing three-compartmental bag of the PN protected it, as stated on the manufacturer's product leaflet.
Third, the possibility of alcoholic Wernicke encephalopathy was entertained, since our patient routinely underwent lock therapy of his central venous catheter with ethanol to prevent bloodstream infection and catheter colonization. Although the volume of ethanol and the technique used ensured that all ethanol was removed from the catheter, not released into circulation, a small amount every time could have been released into the circulation. We ruled out this possibility by measuring blood levels of ethanol prior to and after performing a lock-therapy session; both levels were too low to be detected.
Finally, the caregivers were asked if, during home administration of the PN, vitamin supplementation had been omitted. They denied such a possibility, but it remains a likely cause, since our patient was greatly improved clinically, and considering that even medical staff can underevaluate the significance of vitamin supplementation in these settings, as shown in our case series (
Table 1) [
56789101112], a caregiver is even more likely to underappreciate their importance.
In our case series, thiamine levels, when measured (in half the cases), were below normal levels. This finding reinforces the common practice of initiating treatment with thiamine supplementation when Wernicke encephalopathy is suspected, without waiting for thiamine concentration results or even ordering such a test [
15]. Treatment should be initiated based on presentation and clinical setting, and a brain MRI can be valuable for excluding other causes of neurologic derangement and ophthalmoplegia, but also for confirming the diagnosis [
4].
There are no guidelines for treatment of thiamine deficiency-caused Wernicke encephalopathy in children in terms of dosing, preferred route of administration, duration of therapy, or maintenance therapy. The most frequent therapy includes administration of 100 mg of thiamine intravenously daily (
Table 1) [
56789101112]. However, we achieved similar results—that is, improvement within 48 hours and complete reversion of clinical and imaging findings—with a lower dose of 25 mg of thiamine administered intramuscularly. Overdosing may seem harmless, but given the controversy over whether excessive supplementation can promote tumor growth [
3], we suggest that it may be best to refrain from administering higher dosages.
In conclusion, Wernicke encephalopathy remains a serious complication of certain gastrointestinal diseases, including short bowel syndrome. PN-dependent patients have an increased risk of developing thiamine deficiency. Medical staff and caregivers should be aware of the importance of vitamin supplementation including thiamine. More data is needed for specific treatment recommendation in children; however, high suspicion and immediate parenteral supplementation remain generally accepted practice to prevent serious complications and fatal outcomes.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download