1. Davit-Spraul A, Gonzales E, Baussan C, Jacquemin E. Progressive familial intrahepatic cholestasis. Orphanet J Rare Dis. 2009; 4:1.

2. Cavestro GM, Frulloni L, Cerati E, Ribeiro LA, Corrente V, Sianesi M, et al. Progressive familial intrahepatic cholestasis. Acta Biomed. 2002; 73:53–56.
3. Amer S, Hajira A. A comprehensive review of progressive familial intrahepatic cholestasis (PFIC): genetic disorders of hepatocanalicular transporters. Gastroenterology Res. 2014; 7:39–43.

4. Srivastava A. Progressive familial intrahepatic cholestasis. J Clin Exp Hepatol. 2014; 4:25–36.

5. Gaur K, Sakhuja P. Progressive familial intrahepatic cholestasis: a comprehensive review of a challenging liver disease. Indian J Pathol Microbiol. 2017; 60:2–7.
6. Davit-Spraul A, Fabre M, Branchereau S, Baussan C, Gonzales E, Stieger B, et al. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology. 2010; 51:1645–1655.

7. Arnell H, Nemeth A, Annerén G, Dahl N. Progressive familial intrahepatic cholestasis (PFIC): evidence for genetic heterogeneity by exclusion of linkage to chromosome 18q21-q22. Hum Genet. 1997; 100:378–381.

8. Chen HL, Chang PS, Hsu HC, Ni YH, Hsu HY, Lee JH, et al. FIC1 and BSEP defects in Taiwanese patients with chronic intrahepatic cholestasis with low gamma-glutamyltranspeptidase levels. J Pediatr. 2002; 140:119–124.

9. Hori T, Egawa H, Takada Y, Ueda M, Oike F, Ogura Y, et al. Progressive familial intrahepatic cholestasis: a single-center experience of living-donor liver transplantation during two decades in Japan. Clin Transplant. 2011; 25:776–785.

10. Numakura C, Abukawa D, Kimura T, Tanabe S, Hayasaka K. A case of progressive familial intrahepatic cholestasis type 1 with compound heterozygous mutations of ATP8B1. Pediatr Int. 2011; 53:107–110.

11. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–424.

12. Lykavieris P, van Mil S, Cresteil D, Fabre M, Hadchouel M, Klomp L, et al. Progressive familial intrahepatic cholestasis type 1 and extrahepatic features: no catch-up of stature growth, exacerbation of diarrhea, and appearance of liver steatosis after liver transplantation. J Hepatol. 2003; 39:447–452.

13. Luketic VA, Shiffman ML. Benign recurrent intrahepatic cholestasis. Clin Liver Dis. 1999; 3:509–528. viii

14. Klomp LW, Vargas JC, van Mil SW, Pawlikowska L, Strautnieks SS, van Eijk MJ, et al. Characterization of mutations in ATP8B1 associated with hereditary cholestasis. Hepatology. 2004; 40:27–38.

15. Paulusma CC, Elferink RP, Jansen PL. Progressive familial intrahepatic cholestasis type 1. Semin Liver Dis. 2010; 30:117–124.

16. Maillette de Buy Wenniger L, Beuers U. Bile salts and cholestasis. Dig Liver Dis. 2010; 42:409–418.

17. Sharma A, Poddar U, Agnihotry S, Aggarwal R. A novel truncation mutation in ATP8B1 gene in progressive familial intrahepatic cholestasis. Indian Pediatr. 2016; 53:1099–1101.
18. Bull LN, van Eijk MJ, Pawlikowska L, DeYoung JA, Juijn JA, Liao M, et al. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat Genet. 1998; 18:219–224.

19. Deng BC, Lv S, Cui W, Zhao R, Lu X, Wu J, et al. Novel ATP8B1 mutation in an adult male with progressive familial intrahepatic cholestasis. World J Gastroenterol. 2012; 18:6504–6509.

20. Müllenbach R, Bennett A, Tetlow N, Patel N, Hamilton G, Cheng F, et al. ATP8B1 mutations in British cases with intrahepatic cholestasis of pregnancy. Gut. 2005; 54:829–834.

21. Alvarez L, Jara P, Sánchez-Sabaté E, Hierro L, Larrauri J, Díaz MC, et al. Reduced hepatic expression of farnesoid X receptor in hereditary cholestasis associated to mutation in ATP8B1. Hum Mol Genet. 2004; 13:2451–2460.

22. Jacquemin E, Hermans D, Myara A, Habes D, Debray D, Hadchouel M, et al. Ursodeoxycholic acid therapy in pediatric patients with progressive familial intrahepatic cholestasis. Hepatology. 1997; 25:519–523.

23. Aydogdu S, Cakir M, Arikan C, Tumgor G, Yuksekkaya HA, Yilmaz F, et al. Liver transplantation for progressive familial intrahepatic cholestasis: clinical and histopathological findings, outcome and impact on growth. Pediatr Transplant. 2007; 11:634–640.

24. Egawa H, Yorifuji T, Sumazaki R, Kimura A, Hasegawa M, Tanaka K. Intractable diarrhea after liver transplantation for Byler's disease: successful treatment with bile adsorptive resin. Liver Transpl. 2002; 8:714–716.

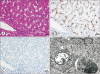
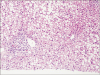
25. Morotti RA, Suchy FJ, Magid MS. Progressive familial intrahepatic cholestasis (PFIC) type 1, 2, and 3: a review of the liver pathology findings. Semin Liver Dis. 2011; 31:3–10.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download