Abstract
The Clinical Mass Spectrometry Research Committee (CMSRC), in affiliation with the Korean Society of Clinical Chemistry (KSCC), conducted a questionnaire survey on opinions about the general status of clinical mass spectrometric analysis in Korea. As a result, we understand that this field has passed through the introductory stage and is settled as a field of clinical laboratory testing in Korea, with the number of new laboratories performing mass spectrometric analysis being low. In spite of the many difficulties in introducing and operating clinical mass spectrometric analysis, there is a strong interest in this field, and even though further expansion is expected, there are still many issues to be resolved. In the future, it will be necessary to make concrete and thorough efforts to further develop the laboratory tests using clinical mass spectrometric analysis in Korea, centering on the CMSRC affiliated with the KSCC.
Go to : 
References
1. Adaway JE, Keevil BG, Owen LJ. Liquid chromatography tandem mass spectrometry in the clinical laboratory. Ann Clin Biochem. 2015; 52:18–38.

2. Antonelli G, Marinova M, Artusi C, Plebani M. Mass spectrometry or immunoassay: est modus in rebus. Clin Chem Lab Med. 2017; 55:1243–5.

3. Hoofnagle AN and Wener MH. The fundamental faws of immunoassays and potential solutions using tandem mass spectrometry. J Immunol Methods. 2009; 347:3–11.
4. Kim B, Lee MN, Park HD, Kim JW, Chang YS, Park WS, et al. Dried blood spot testing for seven steroids using liquid chromatography-tandem mass spectrometry with reference interval determination in the Korean population. Ann Lab Med. 2015; 35:578–85.

5. Han M, Jun SH, Song SH, Park KU, Kim JQ, Song J. Use of tandem mass spectrometry for newborn screening of 6 lysosomal storage disorders in a Korean population. Korean J Lab Med. 2011; 31:250–6.

6. Yu S, Kang ES, Park MH. A questionnaire survey of HLA crossmatch tests in Korea (2015). Lab Med Online. 2017; 7:147–56.

7. Kim CK, Lee H, Yong D, Kim YA. National survey on biosafety in clinical tuberculosis laboratories in Korea. Lab Med Online. 2017; 7:189–95.

8. Lee S, Yu GG, Park KH, Lee SY, Jekarl DW, Yoon NS, et al. Survey of fungal cultures and the identifcation tests used by diagnostic laboratories in Korea. J Lab Med Qual Assur. 2016; 38:143–50.
9. Koh YR, Kim SY, Kim IS, Chang CL, Lee EY, Son HC, et al. Customer satisfaction survey with clinical laboratory and phlebotomy services at a tertiary care unit level. Ann Lab Med. 2014; 34:380–5.

10. de Denus S, Letarte N, Hurlimann T, Lambert JP, Lavoie A, Robb L, et al. An evaluation of pharmacists'expectations towards pharmacogenomics. Pharmacogenomics. 2013; 14:165–75.
Go to : 
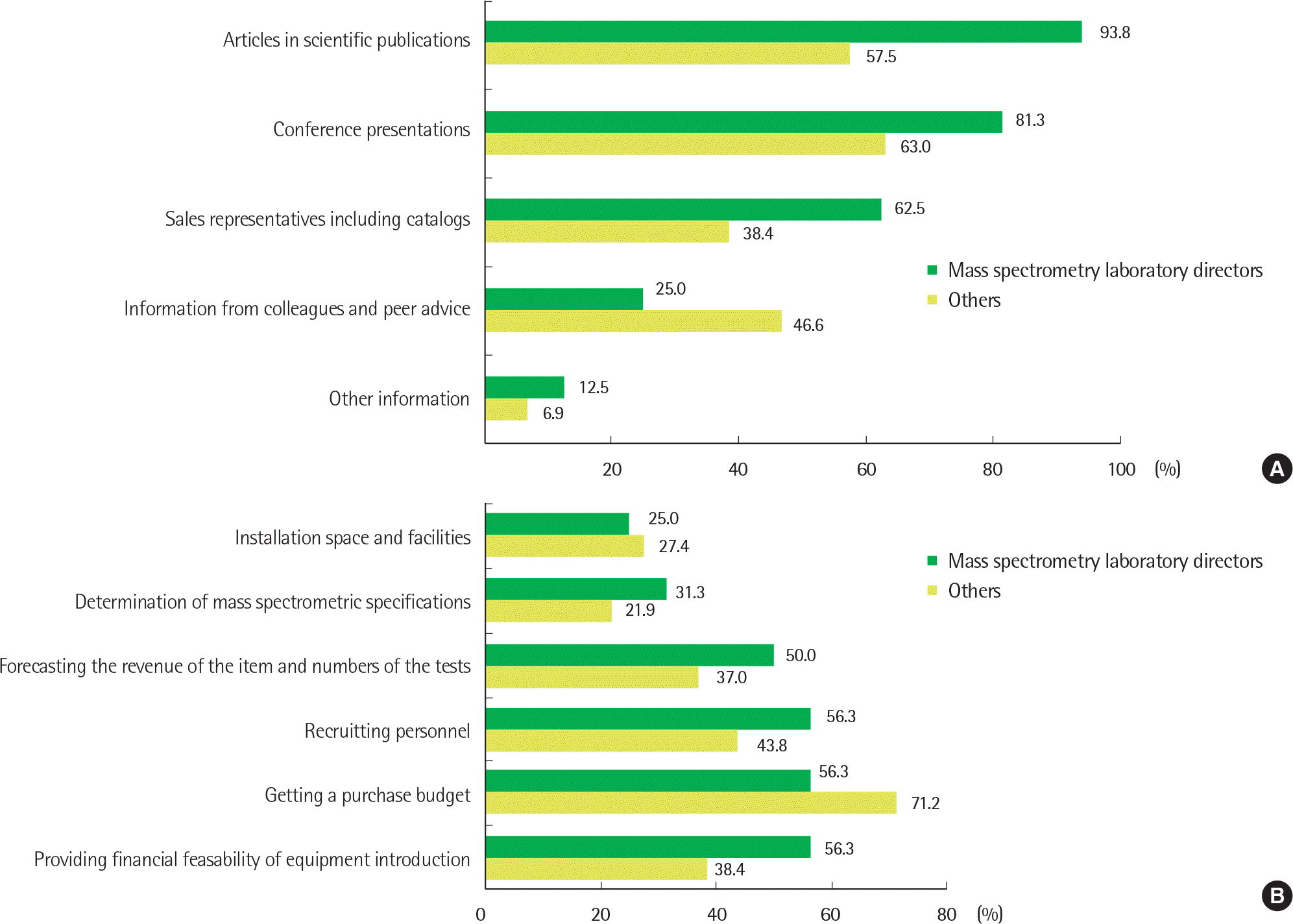 | Fig. 1.General status and opinions about clinical mass spectrometric analysis in Korea. For each question, responders were asked to check up to three items according to priority. (A) What information sources are referenced in relation to mass spectrometric information? (B) What are the difficulties in introducing and purchasing mass spectrometry equipment? (C) Which items would you receive if the Clinical Mass Spectrometry Research Committee conducts a training program about clinical mass spectrometric analysis? (D) What are the difficulties in performing and managing the clinical mass spectrometric analysis? (only applied to the mass spectrometry laboratory directors) Abbreviations: LC, liquid chromatography; LDT, laboratory developed test. |
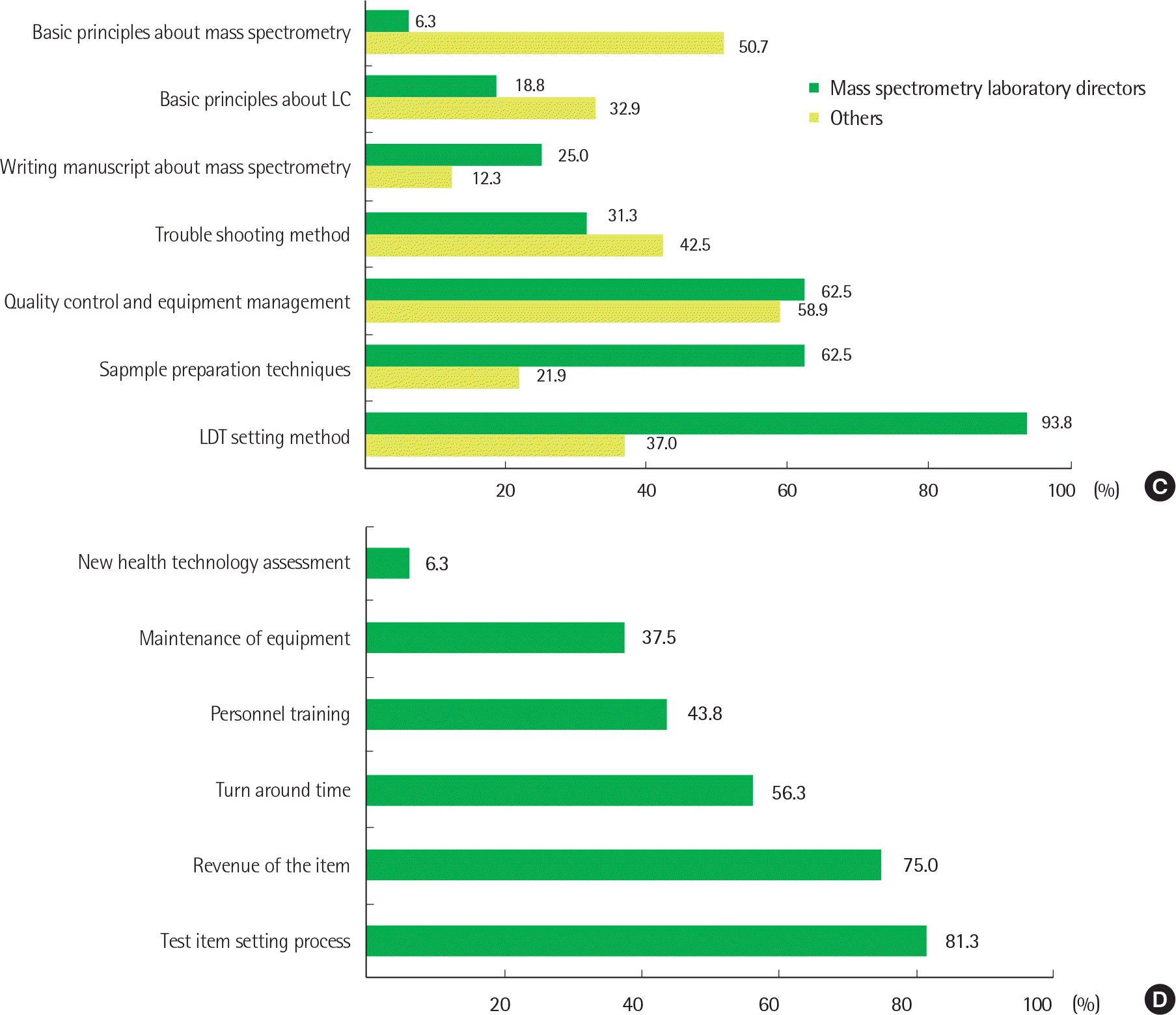 | Fig. 1.General status and opinions about clinical mass spectrometric analysis in Korea. For each question, responders were asked to check up to three items according to priority. (A) What information sources are referenced in relation to mass spectrometric information? (B) What are the difficulties in introducing and purchasing mass spectrometry equipment? (C) Which items would you receive if the Clinical Mass Spectrometry Research Committee conducts a training program about clinical mass spectrometric analysis? (D) What are the difficulties in performing and managing the clinical mass spectrometric analysis? (only applied to the mass spectrometry laboratory directors) Abbreviations: LC, liquid chromatography; LDT, laboratory developed test. |
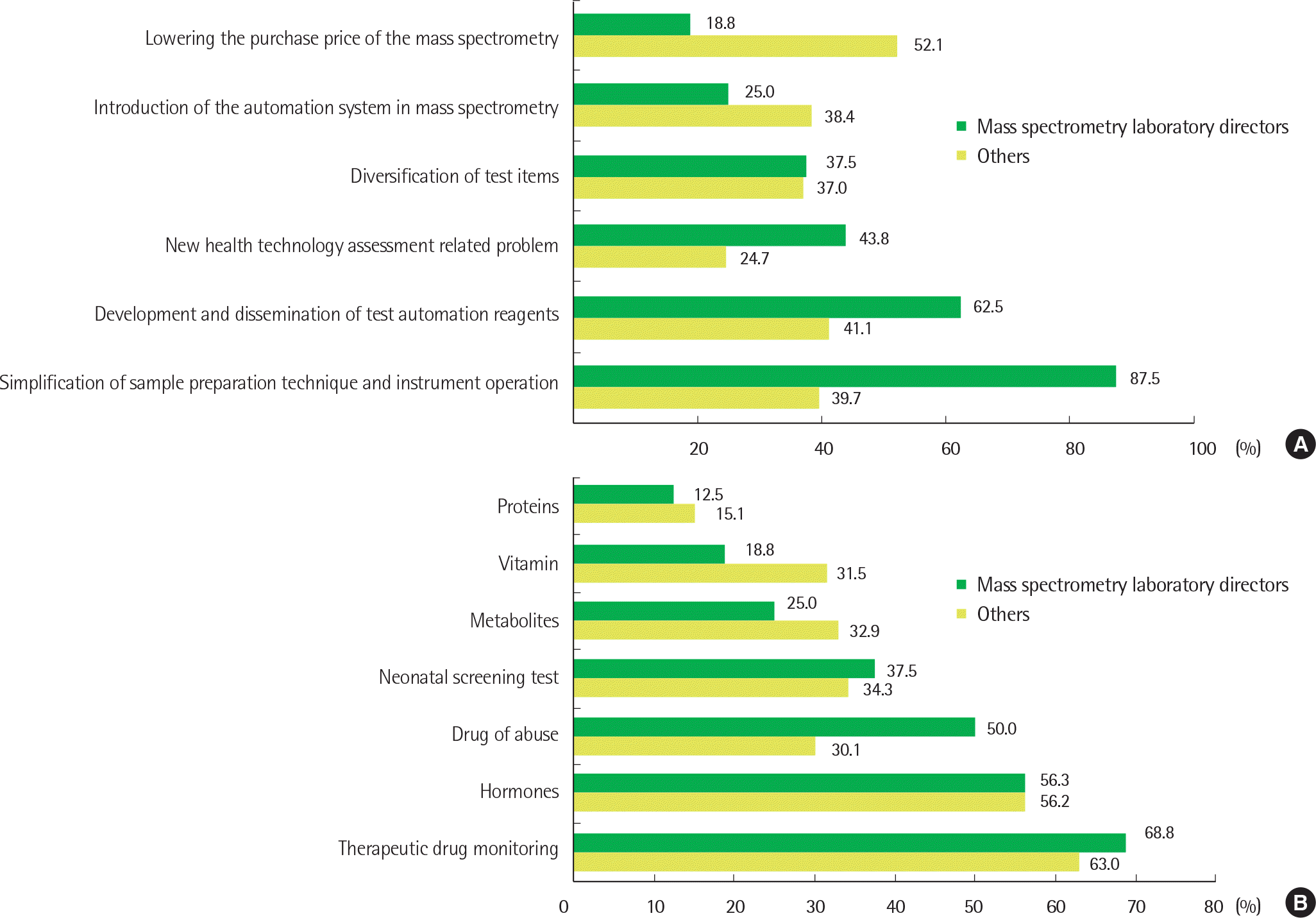 | Fig. 2.Opinions about the prediction of future trends in clinical mass spectrometric analysis in Korea. For each question, responders were asked to check up to three items according to priority. (A) What are the challenges that must be addressed in order to further expand the field of clinical mass spectrometric analysis in the future? (B) Which test items are expected to expand further in the clinical mass spectrometric analysis field in your laboratory in the future? |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download