Abstract
Background
Here we investigated the clinical utilities of blast suspect, large unstained cell (LUC), delta neutrophil index ll (DN ll), and delta neutrophil index l (DN l), analyzed in peripheral blood samples with automated hematology analyzers to predict the relapse of acute leukemia.
Methods
We retrospectively reviewed the medical records of 112 patients, including 56 patients with acute leukemia relapse and 56 controls. Blast suspect, LUC, DN ll, and DN l were compared between the control and leukemia relapse groups.
Results
Significant differences in blast suspect (P<0.001), LUC (P<0.001), DN ll (P<0.001), and DN l (P=0.002) were observed between the leukemia relapse and control groups. The areas under the curve (AUC) value was 0.927 for blast suspect (95% confidence interval [ CI]: 0.8750.978, P<0.001), 0.868 for LUC (95% CI: 0.794–0.941, P<0.001), and 0.900 for DN ll (95% CI: 0.841–0.960, P<0.001). Logistic regression analysis for the prediction of leukemia relapse revealed odds ratio values of 1.52 (95% CI: 1.26–1.96, P=0.0002) for blast suspect, 1.66 (95% CI: 1.27–2.42, P=0.0019) for LUC, 1.16 (95% CI: 1.08–1.29, P=0.0014) for DN ll, and 1.05 (95% CI: 1.01–1.13, P=0.0845) for DN l.
Go to : 
References
1. Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010; 115:453–74.

3. Savani B, Mielke S, Reddy N, Goodman S, Jagasia M, Rezvani K. Management of relapse after allo-SCT for AML and the role of second transplantation. Bone Marrow Transplant. 2009; 44:769–77.

4. Thirup P. LUC, What Is That? Large unstained cells. Clin Chem. 1999; 45:1100.
5. Nahm CH, Choi JW, Lee J. Delta neutrophil index in automated immature granulocyte counts for assessing disease severity of patients with sepsis. Ann Clin Lab Sci. 2008; 38:241–6.
6. Jang MJ, Choi HW, Lee SY, Lee OJ, Kim HR, Shin JH, et al. Application of bone marrow samples for discrimination of acute promyelocytic leukemia from other types of acute leukemia using the routine automated hematology analyzer. Int J Lab Hematol. 2014; 36:531–40.

7. Lee SH, Erber WN, Porwit A, Tomonaga M, Peterson LC. ICSH guidelines for the standardization of bone marrow specimens and reports. Int J Lab Hematol. 2008; 30:349–64.

8. d'Onofrio G and Zini G. Analysis of bone marrow aspiration fuid using automated blood cell counters. Clin Lab Med. 2015; 35:25–42.
9. Buttarello M and Plebani M. Automated blood cell counts: state of the art. Am J Clin Pathol. 2008; 130:104–16.
10. Canovi S and Campioli D. Complete blood counts and automated leucocyte differential results obtained by Siemens ADVIA 2120i in 145 cases of acute leukaemia in adults: insights into cellular pathophysiology and differential diagnosis. Int J Lab Hematol. 2016; 38:e107–10.
11. Yang JH, Kim Y, Lim J, Kim M, Oh EJ, Lee HK, et al. Determination of acute leukemia lineage with new morphologic parameters available in the complete blood cell count. Ann Clin Lab Sci. 2014; 44:19–26.
12. Drewinko B, Bollinger P, Brailas C, Moyle S, Wyatt J, Simson E, et al. Flow cytochemical patterns of white blood cells in human haematopoietic malignancies. I. Acute leukaemias. Br J Haematol. 1987; 66:27–36.
13. Winkel P, Olesen T, Nissen NI. Automated cytochemistry in the prediction of remission following chemotherapy of patients with de novo acute myeloblastic leukemia. Am J Clin Pathol. 1982; 77:50–3.
14. Avnstr⊘m S, Ralfkiar E, Winkel P, Nissen NI. The relative merit of various cytochemical quantities and manual differential count in predicting remission following chemotherapy of patients with de novo acute myeloblastic leukemia. Am J Clin Pathol. 1985; 83:73–6.
15. Gibbs G and Prechtl G. Automated cytochemistry expanded using novel cellular analysis. Int J Lab Hematol. 2015; 37:e133–4.
16. Lanza F, Moretti S, Latorraca A, Scapoli G, Rigolin F, Castoldi G. Flow cytochemical analysis of peripheral lymphocytes in chronic B-lymphocytic leukemia. Prognostic role of the blast count determined by the H∗1 system and its correlation with morphologic features. Leuk Res. 1992; 16:639–46.

17. Vanker N and Ipp H. Large unstained cells: a potentially valuable parameter in the assessment of immune activation levels in HIV infection. Acta Haematol. 2014; 131:208–12.

18. Anderlini P, Przepiorka D, Champlin R, Körbling M. Biologic and clinical effects of granulocyte colonystimulating factor in normal individuals. Blood. 1996; 88:2819–25.

19. Baer MR, Bernstein S, Brunetto VL, Heinonen K, Mrózek K, Swann VL, et al. Biological effects of recombinant human granulocyte colonystimulating factor in patients with untreated acute myeloid leukemia. Blood. 1996; 87:1484–94.

20. Price TH, Chatta GS, Dale DC. Effect of recombinant granulocyte colonystimulating factor on neutrophil kinetics in normal young and elderly humans. Blood. 1996; 88:335–40.

21. Lieschke GJ and Burgess AW. Granulocyte colonystimulating factor and granulocyte-macrophage colonystimulating factor (1). N Engl J Med. 1992; 327:28–35.
Go to : 
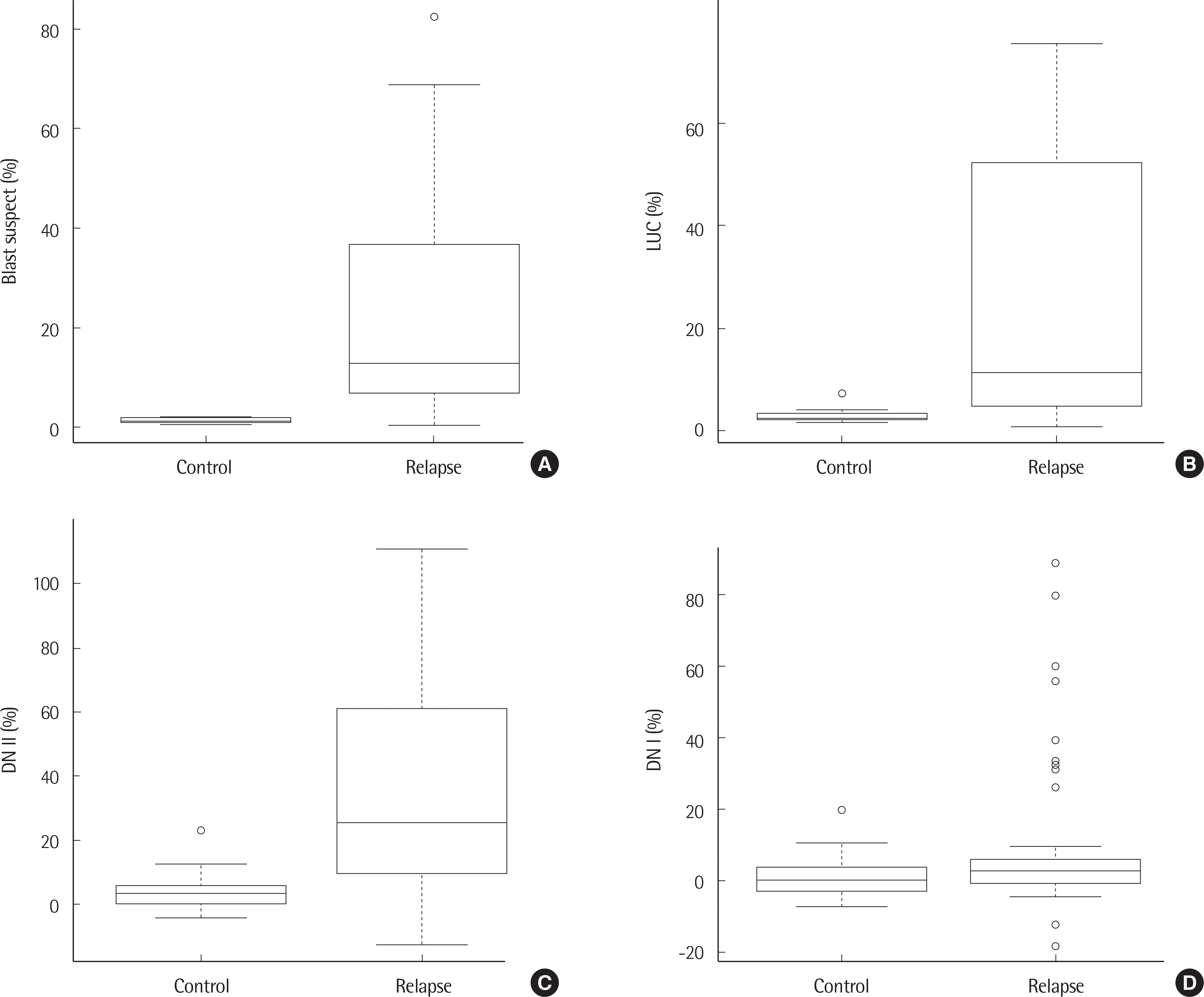 | Fig. 1.Box plot of blast suspect (A), LUC (B), DN II (C), DN I (D) in control and leukemia relapse groups. |
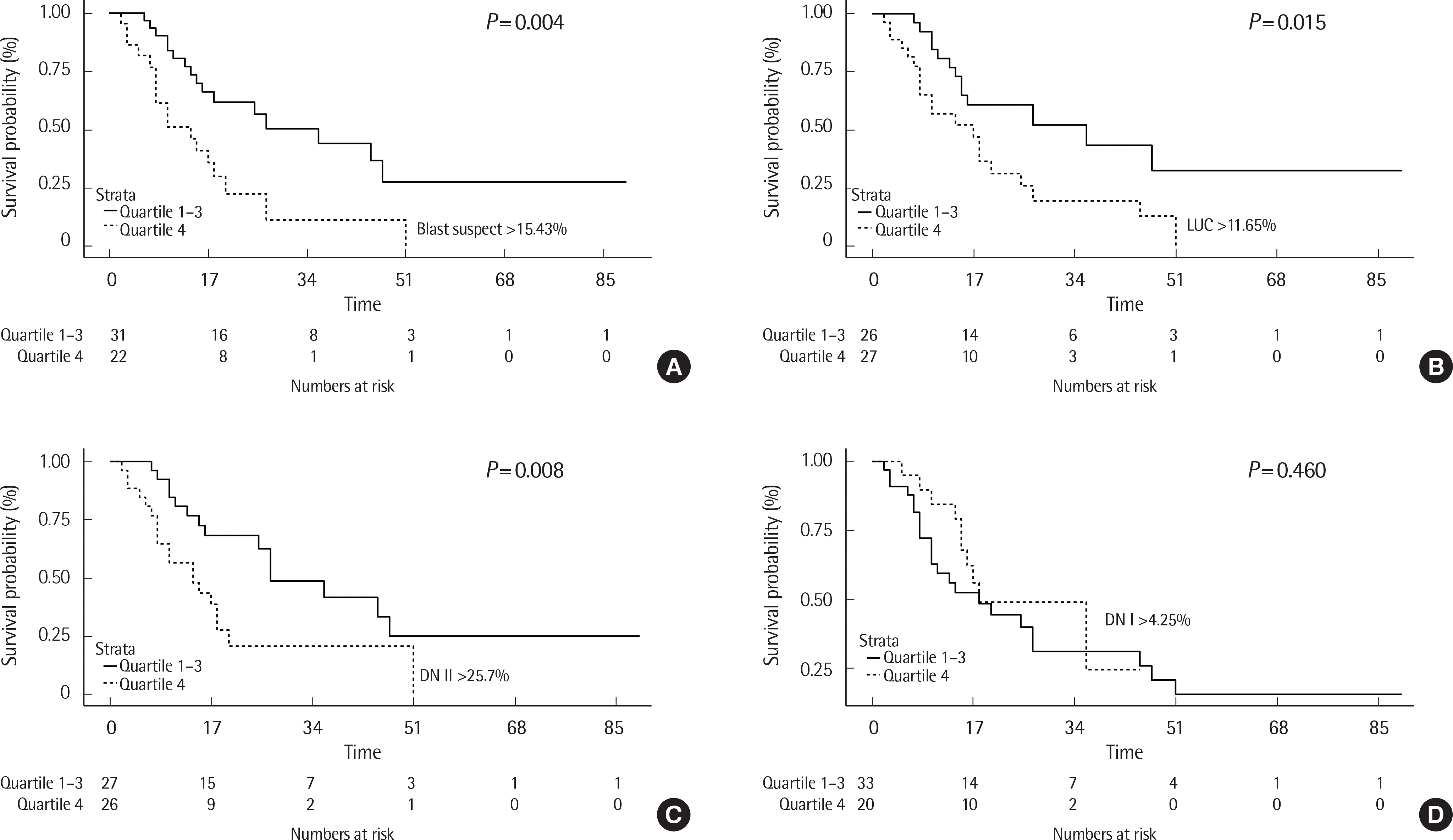 | Fig. 2.Kaplan–Meier overall survival analysis of patients with leukemia relapse with blast suspect (A), LUC (B), DN II (C) and DN I (D). Dotted line represents patients with leukemia relapse in the third quartile (blast suspect >15.43%, LUC >11.65%, DN II >25.7%, DN I >4.25%) and solid line represents patients with leukemia relapse in the first to third quartile. |
Table 1.
Clinical Characteristics of 112 patients
Data are presented as mean±standard deviation or median [IQR]. Abbreviations: IQR, interquartile range; F, female; M, male; BM, bone marrow; PB peripheral blood; DN I, delta neutrophil index I; DN II, delta neutrophil index ll; LUC, large unstained cell; WBC, white blood cell; PLT, platelet; N, normal; T, T lymphoblastic leukemia; MPAL, mixed phenotype acute leukemia; Hb, hemoglobin.
Table 2.
AUC of blast suspect, LUC, DN II, and DN I
Table 3.
Results of univariate logistic regression analysis
| Parameter | OR | 95% CI | P |
|---|---|---|---|
| Blast suspect | 1.52 | 1.26–1.96 | 0.0002 |
| LUC | 1.66 | 1.27–2.42 | 0.0019 |
| DN II | 1.16 | 1.08–1.29 | 0.0014 |
| DN I | 1.05 | 1.01–1.13 | 0.0845 |
Table 4.
Comparison of parameters between control and relapse groups with less than 5% blasts in the peripheral blood




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download