CASE REPORT
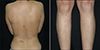
We present 9-year-old fraternal twins (a boy and a girl) with piebaldism, CALMs and intertriginous freckling. The mother of them reported that both of the patients had depigmented macules including hyperpigmented skin areas that were present at birth and showed no changes over time. It was also stated that small brown spots became apparent in the axillary and inguinal folds in infancy and they increased in number and size and spread over the body during childhood. Their physical and mental developments were normal. Family history revealed inheritance of piebaldism on the maternal side. Their mother had also similar findings (
Fig. 1), and she mentioned that her sister and aunt had depigmented macules, frontal white forelock and multiple CALMs (
Fig. 2). However, there were no neurofibromas or other clinical findings of
NF1 in any members of family.
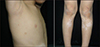
The boy had irregular bordered depigmented macules in different sizes including hyperpigmented skin areas on his ventral trunk, left leg and both arms. He had also numerous CALMs, at least 15 of which were >5 mm in diameter, and bilateral axillary and inguinal freckling (
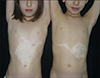
Fig. 3). Similarly, the girl had depigmented macules including islands of hyperpigmented macules on her knees, in the midline of the ventral trunk and on right arm. She had also numerous CALMs, 9 of which were >5 mm in diameter, and bilateral axillary and inguinal freckling (
Fig. 4). We received the patient's consent form about publishing all photographic materials.
No other findings such as poliosis or neurofibromas were detected in neither of the twins. There were no pathologic findings on ophthalmic, neurological, and audiological examinations. Routine laboratory tests, skeletal survey, chest radiography, and magnetic resonance imaging of the temporal bone and brain were normal. Minimal mitral insufficiency was detected in boy and minimal mitral valve prolapse was determined in girl by echocardiography. Genetic testing for NF1 didn't demonstrate NF1 gene mutation in neither of them. Because of the financial reasons genetic testing for KIT and SPRED1 mutations couldn't be performed.
DISCUSSION
Piebaldism is a rare autosomal dominant genodermatosis characterized by congenital depigmentation in affected areas of the skin and/or hair
113. Genetic studies identified that clinical manifestations and phenotypic severity of piebaldism correlate with the type and localisation of the
KIT gene mutation
13. The prevelance of
NF1, another autosomal dominant genodermatosis, is higher than piebaldism. The different clinical features of
NF1 are CALMs, intertriginous freckling, neurofibromas, Lisch nodules, and increased malignant and benign tumors. Based on the National Institutes of Health Diagnostic Criteria. The presence of six or more CALMs over than 5 mm in diameter in prepubertal individuals and intertriginous freckling as in our patients seems to be enough for the diagnosis of
NF1. CALMs and intertriginous freckling are apparent at birth and childhood while the other diagnostic signs are uncommon in small children. Because of the fact that the reliability of the diagnostic criteria improves in advancing ages, the diagnosis of
NF1 especially in small children is difficult to be made in certainty. Therefore, prepubertal patients with CALMs and intertriginous freckling should be closely followed in terms of other findings of
NF11415. Because none of the relatives on the maternal side with piebaldism had other signs of
NF1, the presence of CALMs and the intertriginous freckling in our patients may be a rarely seen clinical variant of piebaldism rather than the diagnostic criteria of
NF1.
Piebaldism is characterized by the depigmented macules of the skin owing to the absence of melanocytes while,
NF1 is characterized by hyperpigmented macules, intertriginous freckling and pigmented iris hamartomas depending on the increased number of melanocytes and macromelanosomes. Piebaldism is associated with the mutations of the
KIT (receptor tyrozine kinase) gene on chromosome 4q12 or the SLUG (zinc finger neural crest transcriptional factor) gene on chromosome 8q11, whereas
NF1 is associated with mutations of the
NF1 gene on chromosome 17q11.2. Inactivating mutations or deletions of the
KIT or SLUG genes result in decreased receptor tyrosine kinase signaling and a decrease in melanogenesis, but the mutations or the deletions of the
NF1 results in enhanced receptor tyrozine kinase signaling. In some studies, increased
KIT expression and activity have also been demonstrated on
NF1-associated neurofibromas
69.
The absence of other clinical findings of
NF1 in patients with similar phenotypes and the absence of
NF1 mutations in a small number of patients undergone genetic testing suggest that these findings are a rare phenotype of piebaldism. Recently, Spritz
10 claimed that multiple CALMs and intertriginous freckling occur frequently in the typical patients of piebaldism. Spritz
10 also reported that these hyperpigmented macules are the minor clinical diagnostic criteria for
NF1. For this reason it has been suggested that in such patients the diagnosis of
NF1 must be based on the nonpigmentary diagnostic criteria (e.g., neurofibromas) and demonstration of a mutation in
NF1 gene by DNA testing
10. Subsequently, Duarte et al.
11 suggested that there is no definition reported by Spritz as “minor clinical diagnostic criteria for
NF1” and that distinction is only a personal opinion. It has been also claimed that occurrence of neurofibromas, which are mentioned as necessary for the diagnosis of
NF1, is not possible in these patients with piebaldism due to the
KIT gene mutation. Although the sensitivity of the molecular recognition of
NF1 is 95%, it has also been reported to be impossible to detect microdeletions which accounts for 5%~10% of
NF1 mutations. Therefore, it has been thought that
NF1 diagnosis cannot be excluded in these patients and a possible
NF1 diagnosis should not be missed in order to prevent systemic complications in later periods
11.
Although comprehensive genetic analyses were not performed in the prior reported patients,
KIT gene mutation has been detected in subsequent five patients while there was no
NF1 gene mutation in any of them
678. Recently, the loss of function of the
SPRED1 (Sprouty-related, Ena/vasodilator-stimulated phosphoprotein homology-1 domain containing protein 1) gene associated Legius syndrome with
NF1-like clinical findings has also been investigated in three patients with piebaldism, multiple CALMs and intertriginous freckling
78. Chiu et al.
7 identified a noval heterozygous mutation in the
KIT gene, resulting in an aminoacid substitution in the intracellular tyrosine kinaz domain, in a 5-year-old girl. It has been thought that this different mutation in the
KIT gene leads to inadequate phosphorylation of
SPRED1 gene on chromosome 15q, resulting loss of inhibition of the ras/MAPK pathway, with multiple CALMs and clinical phenotype similar to Legius syndrome
7. The other features of Legius syndrome include lipomas, macrocephaly and neurocognitive disorders. Although the patients with Legius sydrome meet the diagnostic criteria for
NF1, they do not need the careful clinical follow-up for
NF1 because of no neurofibromas, Lisch nodules and central nervous system tumors are seen
78. In the other two patients reported by Stevens et al.
8, complex
KIT gene mutations were defined whereas no mutation was detected in the
NF1 and
SPRED1 genes.
Consequently, it has been determined that all patients regarded as coexistence of piebaldism and
NF1 had no other findings of
NF1 except one patient and there was no mutation of
NF1 gene in genetically analyzed patients. In addition to this, as in our patients, it is not possible to exclude a possible diagnosis of Legius syndrome in these patients, because the
SPRED1 gene mutation analysis was not performed in the majority of prior reported ones. Chiu et al.
7 claimed that the coexistence of piebaldism and Legius sydrome would be much less than the association of
NF1 because both
KIT and
SPRED1 gene mutations are very rare and each is on different chromosomes. However, the possibility of these recently reported mutations in our patients cannot be excluded due to the lack of comprehensive genetic testing.
As a summary, we identified two patients from a family with piebaldism, associated with multiple CALMs and intertriginous freckling. We consider that this clinical presentation is an uncommon phenotypic variant of piebaldism due to absence of NF1 mutation and the other features of NF1 disease or Legius syndrome. For this reason, we think that these patients do not need a careful clinical follow-up and further examination for NF1. Besides, it may be suitable that these individuals with piebaldism showing a clinical phenotype similar to NF1 or Legius syndrome should be further tested for KIT and SPRED1 gene mutations.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download