INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory skin disease that is caused by a combination of genetic, environmental, and immunological factors
1. Immunologically, it is characterized by the T helper type 2 (Th2) immune response. When allergens and antigens enter through the damaged skin, Langerhans cells and keratinocytes secrete cytokines and chemokines, including interleukin (IL)-12 and IL-18, and chemoattract various immune cells
1. In the acute stage of AD, Th1, Th2, Th17, and Th22 immune responses are all present, with the Th2 immune response mainly contributing to the pathogenesis; this leads to the increased production of IL-4, IL-5, and IL-13
2. Recently, it was demonstrated that the proportion of Th17 cells were increased in peripheral blood mononuclear cells of patients with AD and were infiltrated more markedly in the acute lesions than in the chronic lesions of AD
3. IL-17 plays a role in keratinocyte stimulation to produce vascular endothelial growth factor, tumor necrosis factor-α, IL-8, granulocyte-macrophage colony-stimulating factor, and chemokine (C-X-C motif) ligand 10. In contrast, in chronic AD, the production of IL-4 and IL-13 is reduced and the production of IL-5 and IL-12 is increased, suggesting that Th1 cells are predominant in the chronic lesions of AD
2. Interferon secreted by Th1 cells causes keratinocyte apoptosis, skin remodeling, and hypoxia in Th22 cells
1.
Most of the patients with AD (80%~90%) have been reported to have high serum immunoglobulin E (IgE) levels
4. Although the role of IgE in the pathogenesis of AD has not yet been fully understood
1, it has been reported that serum IgE levels can be used as a parameter of severity for AD
4.
Treatment of AD includes moisturizers, steroids and immunosuppressive agents, narrow-band ultraviolet B (NB-UVB) therapy, and biologics, and the treatment focuses on improving skin barrier function and controlling the immune system
5. However, systemic medicines have various side effects, especially when used for long periods of time. In the case of NB-UVB, it presents disadvantages such as requiring frequent hospital visits. Recently, dupilumab has been developed and used in the treatment of severe AD as it inhibits IL-4 and IL-13 signaling
6. In Korea, however, dupilumab is not easily accessible as it is expensive and is not yet covered by health insurance.
Glucosamine (2-amino-2-deoxy-D-glucose; GlcN) is an amino sugar synthesized from glucose by the hexosamine biosynthetic pathway that is present in cells of the body and has anti-inflammatory and immunomodulatory effects
7. Previous studies have reported the efficacy of GlcN in the treatment of osteoarthritis and in improving the symptoms of rheumatoid arthritis
89. A recent study demonstrated that GlcN has anti-allergic effects in allergic asthma and rhinitis animal models
7.
We expected that GlcN, which inhibits the Th2 immune response, may be effective in the treatment of AD. We conducted this study to investigate the therapeutic effect of GlcN by performing clinical and histopathological examination, as well as measuring the concentrations of tissue IL-4, IL-13, and IL-17 and serum total IgE levels.
MATERIALS AND METHODS
AD animal model and subjects
This study was carried out in female BALB/c mice (Orient Bio Inc., Seongnam, Korea) aged 8 weeks with AD induced by ovalbumin (OVA; Sigma-Aldrich Korea, Yongin, Korea). Twenty-five BALB/c mice were divided into five groups of five mice: control group without induction of AD (group A), AD group with null treatment by phosphate-buffered saline (PBS) (group B), and AD groups treated with 10 mg, 20 mg, and 40 mg of GlcN (Sigma-Aldrich Korea) administration (groups C, D, and E, respectively). For the AD induction groups (groups B~E), 1.5 ml of OVA and 3 ml of aluminum hydroxide gel (Thermo Fisher Scientific, Waltham, MA, USA) were mixed, and 150 µl of the mixture was intraperitoneally injected into the mice three times a week for three weeks. After a week of OVA injection, mice were epicutaneously sensitized with OVA patches. The patches were made by dripping 50 µl of OVA (1 mg/ml) in a gauze (1×1 cm) which was placed on the back skin three times per week for two weeks. Group A was injected with PBS instead of OVA. This experiment was conducted in specific-pathogen-free environment and the mice received an OVA-free diet. This study was reviewed and approved by the Institutional Animal Care and Use Committee of Inha University (INHA 181120-601).
Administration of glucosamine
After AD was induced, we injected 100 µl of GlcN intraperitoneally at concentrations of 1 mg/10 µl, 1 mg/5 µl, and 1 mg/2.5 µl into the mice of groups C, D, and E, respectively three times per week for three weeks. After a week of GlcN administration, OVA patches were attached three times for one week. For groups A and B, 100 µl of PBS was administered instead of GlcN (
Fig. 1).
Clinical dermatitis scores
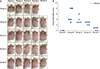
Two dermatologists examined the skin of the AD-induced mice after GlcN administration. The skin condition was scored from 0 to 3 for erythema, dryness, excoriation, and edema: 0 (absence), 1 (mild), 2 (moderate), and 3 (severe). Clinical dermatitis scores were defined as the sum of all scores.
Measurement of concentration of cytokine in tissue
Biopsy specimens from the epidermis to the subcutaneous layer were frozen in liquid nitrogen and stored at −80℃. Then, the specimens were added with RIPA buffer (Pierce Biotechnology, Rockford, IL, USA) supplemented with protease inhibitor (Pierce Biotechnology) and were homogenized on ice with a micro tissue homogenizer (DWK Life Sciences, Millville, NJ, USA). The homogenized specimens were centrifuged and the supernatant was used to measure IL-4, IL-13, and IL-17 levels with individual enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) following the manufacturer's instructions.
Measurement of total IgE in serum
Blood samples were obtained before and after GlcN administration in all groups. Blood (50 µl) was collected from the orbital vein after AD induction and from the heart after GlcN administration. The serum was separated and IgE levels were measured using a BD OptEIA Mouse IgE ELISA kit (Pharmingen, San Diego, CA, USA) according to the manufacturer's instructions.
Histopathological analysis
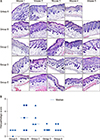
After GlcN administration, Tiletamine/Zolazepam (0.008 ml/10 g) and Xylazine (0.002 ml/10 g) were injected for general anesthesia. Then, skin biopsy was performed. Specimens were fixed in 10% formaldehyde and embedded in paraffin. The tissue sections were stained with hematoxylin and eosin (H&E). Severity of inflammation in the dermis was evaluated grossly in H&E slides by scoring on a scale from 0 to 5: 0 (none), 1 (mild), 2 (mild to moderate), 3 (moderate), 4 (moderate to severe), and 5 (severe).
Statistical analysis
Jonckheere–Terpstra test was performed to determine the likelihood of changes in clinical dermatitis scores, concentrations of tissue cytokines, and serum total IgE levels according to the GlcN dose. Comparison of Serum IgE level between before and after GlcN or PBS administration for each groups were performed by Wilcoxon signed rank test. Fisher's exact test was performed to compare histopathological scores among groups. Statistical analysis was performed using PASW Statistics ver. 18.0 (IBM Corp., Armonk, NY, USA). A value of p<0.05 was considered statistically significant.
DISCUSSION
In this study, we found that GlcN was clinically and histologically effective in improving AD and inflammation, as well as in decreasing the concentrations of tissue IL-13, IL-17, and serum total IgE.
GlcN reduced clinical dermatitis scores and showed a gross decrease in infiltration of inflammatory cells. Recently, there have been studies on the effects of GlcN in various allergic diseases. Previously, a study using NC/Nga mice with
Dermatophagoides farina (Df)-induced AD-like skin lesions also reported that GlcN treatment reduced clinical dermatitis scores
10. Another study showed that crystallinity controlled N-acetyl glucosamine (CCG) reduced histamine release, IL-1β production, and caspase-1/nuclear factor-κB (NF-κB) activation in human mast cell line-1 cells, and demonstrated the anti-allergic and anti-inflammatory effects of GlcN
11. They also reported that CCG reduces systemic anaphylaxis, ear swelling, and passive cutaneous anaphylaxis in a mouse model
11.
In this study, the concentrations of tissue IL-13 and IL-17 were decreased after GlcN administration, but tissue IL-4 levels did not change. Previously, an in vitro study showed that GlcN inhibits the secretion of IL-5 in primary mixed lymphocyte cultures (MLC) using splenocytes from C57BL/6 or BALB/c mice and inhibits IL-4 and IL-5 secretion in secondary Th2-polarized MLC
12. Another study using NC/Nga mice AD models showed that Th2 cytokines including IL-4 and IL-5 were significantly reduced by GlcN treatment, in contrast to our results
10. We supposed that this is due to differences in experimental methods. They injected GlcN once a day and induced AD using Df body ointment, while we injected GlcN three times per week and induced AD using OVA. In addition, they measured cytokine levels in the spleen, while we measured cytokine levels using skin biopsy specimens. A study using BALB/c mouse allergic asthma and rhinitis models reported that IL-4, IL-5, IL-6, IL-17, and eosinophil counts decreased in the bronchoalveolar lavage fluid after GlcN administration, and that GlcN inhibits Th2 and Th17 cytokines
7.
We also found that serum IgE levels tended to be lower after GlcN administration. A previous study using NC/Nga AD mouse models also reported that GlcN reduced serum IgE levels, similar to our results
10. Another study using allergic rhinitis and asthma mice models also evaluated serum total IgE and OVA-specific IgE levels and reported a decrease in both IgE levels after GlcN administration
7. Serum total IgE levels before and after treatment were elevated in all groups including group A. We considered it the characteristic of the mouse. Previously, a study using inbred BN-rats showed that total serum IgE levels increased from the age of 1 month to the age of 5 months
13.
GlcN showed a decrease in infiltration of inflammatory cells. A previous study using NC/Nga mice with Df-induced AD-like skin lesions reported that GlcN reduced infiltration of dermal inflammatory cells, including mast cells and eosinophils
10. In another study using allergic asthma and rhinitis BALB/c mouse models, GlcN showed anti-allergic effects and decreased the infiltration of inflammatory cells in lung and nasal tissues
7.
GlcN regulates factors involved in inflammation and the NF-κB pathway, and inhibits the Th2 immune responses
14. Exogenous GlcN enters the cell through the glucose transporters (GLUT-1, GLUT-2, and GLUT-4) and increases intracellular UDP-N-acetyl-GlcN (UDP-GlcNAc)
15. UDP-GlcNAc is involved in the O-GlcNAcylation of nuclear and cytoplasmic transcription factors, as well as lipopolysaccharide-related inflammatory factors
715. In addition, GlcN prevents IκB degradation and decreases NF-κB nuclear accumulation and NF-κB reporter activity
16. It is suggested that GlcN mediates its anti-inflammatory effects via this mechanism. In addition, GlcN regulates the antigen presentation of dendritic cells, and inhibits the proliferation of CD4
+ T cells and the secretion of Th2 cytokines such as IL-4 and IL-5
12.
This study has some limitations. First, our experimental model is an animal model with AD-like skin lesions, and thus does not fully represent true AD. Therefore, further studies are needed to demonstrate the effect of GlcN in humans with AD. Second, the sample size is small. Third, the optimal dose of GlcN is unknown. Finally, there was no positive control to compare the effect of GlcN. In further studies, it would be desirable to conduct experiments with a positive control such as cyclosporine or a systemic steroid.
In conclusion, GlcN improved AD-like skin lesions and decreased tissue IL-13 and IL-17, as well as serum total IgE levels in an animal model. These results may be a basis for the future use of GlcN as a therapeutic agent for AD.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download