Abstract
Local anesthetic systemic toxicity (LAST) refers to the complication affecting the central nervous system (CNS) and cardiovascular system (CVS) due to the overdose of local anesthesia. Its reported prevalence is 0.27/1000, and the representative symptoms range from dizziness to unconsciousness in the CNS and from arrhythmias to cardiac arrest in the CVS. Predisposing factors of LAST include extremes of age, pregnancy, renal disease, cardiac disease, hepatic dysfunction, and drug-associated factors. To prevent the LAST, it is necessary to recognize the risk factors for each patient, choose a safe drug and dose of local anesthesia, use vasoconstrictor , confirm aspiration and use incremental injection techniques. According to the treatment guidelines for LAST, immediate application of lipid emulsion plays an important role. Although lipid emulsion is commonly used for parenteral nutrition, it has recently been widely used as a non-specific antidote for various types of drug toxicity, such as LAST treatment. According to the recently published guidelines, 20% lipid emulsion is to be intravenously injected at 1.5 mL/kg. After bolus injection, 15 mL/kg/h of lipid emulsion is to be continuously injected for LAST. However, caution must be observed for >1000 mL of injection, which is the maximum dose. We reviewed the incidence, mechanism, prevention, and treatment guidelines, and a serious complication of LAST occurring due to dental anesthesia. Furthermore, we introduced lipid emulsion that has recently been in the spotlight as the therapeutic strategy for LAST.
Go to : 
An ideal local anesthetics should be potent, reversible, low cost, stable, and easy to metabolize and emit. Furthermore, it should have rapid onset action, suitable duration of effect, and good tissue penetration. Another one of the important qualifications is that there should be no adverse effect, neither local nor systemic. Complications due to local anesthesia can be divided into three major areas; complications associated with vasoconstrictor, needle, and absorption of local anesthetics. Complications associated with the added vasoconstrictor include elevated blood pressure and increased heart rate. Another complications associated with the needle include syncope, hematoma, pain, edema, infection, paresthesia, nerve paralysis, and breakdown of needle or cartridge. Absorption of the local anesthetics include local reaction, idiosyncrasy, toxicity, and allergy or anaphylactic reaction [1]. In this article, we have focused on local anesthetic systemic toxicity (LAST), which refers to systemic toxicity rather than local reactions due to local anesthetics, and the usage of lipid emulsion for treatment of LAST. Lipid emulsion is mainly used as parenteral nutrition for patients in intensive care units, but it has recently been widely used as a non-specific antidote in various types of drug toxicity, including that of local anesthesia [2].
Paul et al. have described the toxicity caused by overdose of local anesthetics during dental treatments and the pharmacokinetic progression, and reported the importance of drug selection and safety levels of the drugs [3]. For the first time, Ciechanowicz et al. reviewed the use of lipid emulsion in the treatment of LAST and its effect in dentistry [4]. In this study, we aimed to review the predisposing factors of LAST in dentistry and present the effectiveness and mechanism of lipid emulsion as per the guidelines of this treatment.
Go to : 
LAST refers to the complication particularly affecting the central nervous system (CNS) and cardiovascular system (CVS) due to the overdose of local anesthetics [5678]. LAST is a life-threatening complication and reportedly occurs in 0.03% people (0.27/1,000) [91011]. To elaborate, the order of incidence of complications from highest to lowest based on the type of block is penile, local infiltration, neuraxial, upper extremity, paravertebral, lower extremity, head and neck, topical, transversus abdominis plane, and intravenous block. Penile blocks are most commonly associated with complications, because of the large distribution of blood vessels [12]. This is followed by local infiltration, which is commonly used in dentistry. The order of incidence of complications from highest to lowest according to the local anesthetics used is bupivacaine, lidocaine, ropivacaine, mixture local anesthetics, levobupivacaine, chloroprocaine, and articaine. Lidocaine, the most commonly used anesthetic in dentistry, accounted for approximately 25% of complications reported. The high incidence of complications of lidocaine, which is comparative safe, could be due to its high frequency of use [1314151617]. If the local anesthetics has been mainly injected directly into the blood vessel, LAST appears immediately after injection; whereas, if the anesthetic is absorbed from the blood vessels into the tissues, LAST appears over time after injection [18192021].
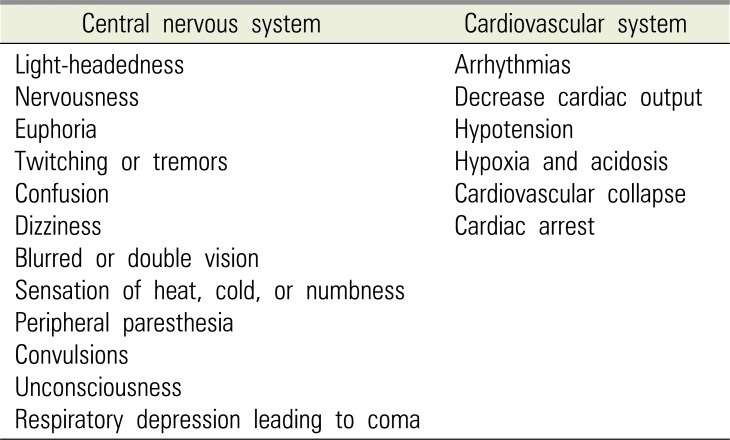
LAST is a fatal complication caused by overdose of local anesthesia; therefore, when symptoms occur, it should be recognized immediately. The American Society of Anesthesiologists recommends standard monitoring (electrocardiogram, blood pressure, oxygen saturation) for immediate recognition. It is helpful to talk to the patient often and check for symptoms indicating LAST [22]. The symptoms of fatal LAST occurring due to local anesthesia in dentistry can be divided into the CNS symptoms and CVS symptoms (Table 1) [4].
Because of the wide spectrum of symptoms related to the CNS, the adverse effects can be recognized quickly. The generalized function of local anesthetics is generally related to blood or plasma concentration. The clinical symptoms are getting stronger with higher concentrations. If the blood concentration of local anesthetics increases to 4.0–7.0 µg/L, symptoms of CNS excitation become clearer. Symptoms such as increased speech, altered mental state, anxiety, tinnitus, loss of direction, and loss of perception are observed. Following these clear symptoms of CNS excitation, a corresponding inhibition phase follows. Here, a patient may show symptoms of lethargy, loss of reaction, loss of limb motion, sleep, drowsiness, and weakness. If plasma concentration increases to 7.5–10 µg /mL, a whole-body seizure can occur [9232425]. The CNS is known as the system most sensitive to high concentrations of local anesthetics in blood. One reason for this is that all local anesthetics can penetrate the blood brain barrier (BBB). Particular attention should be paid to females of the childbearing age, because local anesthetics can pass through the placenta as well as the BBB. Local anesthetics have anticonvulsant effects at low concentrations, but may present CNS symptoms in two phases at high concentrations. Depressive phase symptoms (respiratory depression & coma) appear after the excitatory phase symptoms (tremors & convulsions) [26].
While the effect of local anesthetics on the CNS is inhibition or excitation depending on the concentration, the CVS toxicity is marked by inhibition. CVS toxicity includes conduction disturbances, myocardial dysfunction, changes in the peripheral vascular tone, and cardiac arrest. The first reaction to CVS toxicity is cardiac rhythm disturbance. Regular conduction is hindered by the sodium channel blockade, causing changes in the cardiac rhythm. At high concentrations, this can directly inhibit the myocardium and slow the conduction through the Purkinje fibers, thus lengthening the refractory period. Myocardial dysfunction occurs due to various mechanisms. Calcium channels and sodium-calcium exchange pump blockade are reduced to preserve calcium, ultimately reducing contractility. Local anesthetics at a concentration of ≥5.0 µg/mL show inhibitory effects on the heart functions in proportion to the dose. At a concentration of ≥10.0 µg/mL, serious circulatory collapse and asystole occur. When a cardiac arrest occurs, aggressive cardiac resuscitation is mandatory because there is no permanent damage to the heart. Complete recovery of cardiac function is possible if the effect of LAST disappears and complications are properly treated [31227282930].
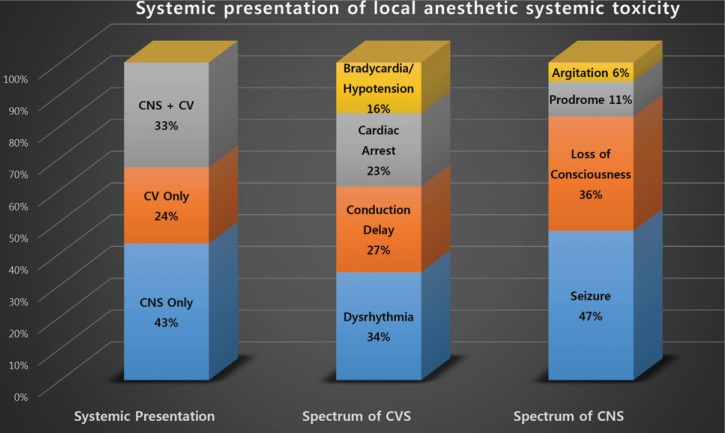
Although approximately 40% of LAST occurs non-specifically [27], CNS depression, especially loss of consciousness and seizures, are the most common (68–77%) characteristics of LAST [1131]. According to the findings, LAST starts with changes seen in the CNS, and 1/3 cases show concurrent CVS symptoms, while 1/4 cases only present CVS disturbances [11] (Fig. 1). While most incidences of LAST occur immediately after local anesthetics administration, recently published studies have reported cases of symptoms occurring few hours or days after the infusion of the drugs. Thus, more detailed and continued monitoring is necessary [3233].
There are two predisposing factors for LAST: patient factors and drug factors. Patient factors include age, weight, consuming other medicines simultaneously, presence of other diseases, genetic factors, and mental status. Therefore, recording medical history before local anesthesia administration is required. It may be helpful to have the questionnaire written before the treatment and using it to collect the necessary information.
Some view age as the biggest risk factor in LAST [11]. Because neonates and infants have small number of enzymes decomposing local anesthetics, 15% reduced dose is injected in young infants <4 months old. In elderly patients, drug accumulation increases due to the decrease in the metabolic organ perfusion and pharmacodynamic functions. Moreover, because of the reduction of skeletal muscle mass, which is a reservoir of local anesthetics, the risk of LAST increases in older patients. Thus, it is recommended to use 10–20% reduced doses of the recommended dose [34]. In pregnant patients, high concentration of local anesthetics in blood is observed due to reduced α-1 acid glycoprotein and increased cardiac output. Due to the combined effects of several factors, a reduction in the local anesthetics dose is recommended [35]. Because patients with renal disease, cardiac disease, or hepatic dysfunction have slower metabolism and delayed elimination of local anesthetics, there is increased plasma concentration and hence reduction of dose is recommended [1536373839].
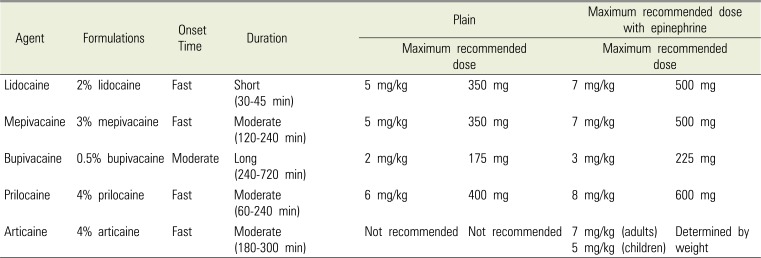
Drug factors include concentration of drug, dosage, route of administration, vascular distribution at the injection site, vasoconstriction, and pharmacokinetics factor. Local anesthetics currently sold in South Korea are amide-type (lidocaine, mepivacaine, bupivacaine, prilocaine, and articaine) and ester-type (procaine and tetracaine). The types, functions, and recommended doses of dental local anesthetics currently being sold in South Korea are summarized in Table 2 [4041]. Amide-type agents, especially lidocaine, are commonly used in most dental settings because they have the advantages of being fast-acting, causing fewer complications, and being reliable. Vasodilation is one of the pharmacokinetic characteristics of all local anesthetics, except cocaine. Hence, uptake is easy and the risk of LAST increases. Therefore, most dental local anesthetics are used along with a vasoconstrictor to decrease complications and increase duration. The blood concentration of local anesthetics influences the occurrence of LAST. There is a high tendency for LAST to occur if the local anesthetics are injected directly into the blood vessels, the injected dosage is high, the drug is fast absorbing, or the drug slows metabolism or excretion. Moreover, local anesthetics with high lipid solubility (e.g., bupivacaine) usually cause cardiovascular collapse more commonly than local anesthetics with low lipid solubility (e.g., lidocaine). Cardiac arrest due to bupivacaine toxicity, which is the most lethal toxicity due to local anesthetics, does not respond to cardiopulmonary resuscitation (CPR) [42].
To prevent LAST, appropriate plasma concentration of local anesthetics is important, and hence the safe dosage of each local anesthetics should be known along with the risk factors of each patient must be assessed before injecting the appropriate dose (Table 2). Although the threshold of plasma concentration may differ in each patient [43], the doses in normal patients and in those with risk factors must be differentiated.
The dentists must also select the appropriate drug carefully. Because highly lipid-soluble bupivacaine has a tendency to induce a cardiovascular collapse [42], it must be used cautiously where other drugs cannot be used (e.g., lidocaine allergy). As levobupivacaine and ropivacaine show similar pharmacokinetics as bupivacaine, the safe dosage must be noted (2.0 mg/kg for bupivacaine, 2.5–3.0 mg/kg for levobupivacaine, and 3.0–4.0 mg/kg for ropivacaine) [4]. It is recommended that these drugs be avoided if there is a high possibility of intravascular injection, such as in the case of the inferior alveolar nerve block. There are also other prevention methods, such as needle aspiration, incremental injection (60 seconds or more for 1 mL injection), and use of vasoconstrictors.
It is most important to predict the risk factor and prevent the occurrence of LAST. Patient factors, such as old or young age and pregnancy must be understood and a dose lower than the recommended dosage must be injected. The injection should be slow at a rate of ≤ 1 mL/minute. During each injection, aspiration must be performed to avoid intravenous injection. Unless the patient has some serious cardiovascular disease, a vasoconstrictor must be used along with the local anesthetics. The choice for local anesthetics is equally important. Using lidocaine or articaine, which have relatively lower toxicity, instead of local anesthetics with relatively high lipid-solubility (e.g., bupivacaine) can help prevent LAST.
Go to : 
Association of Anaesthetists of Great Britain and Ireland (AAGBI) and the American Society of Regional Anesthesia and Pain Medicine (ASRA) published safety guidelines on the toxicity of local anesthetics in 2010. Updated versions have been published by ASRA in 2012 and 2017.
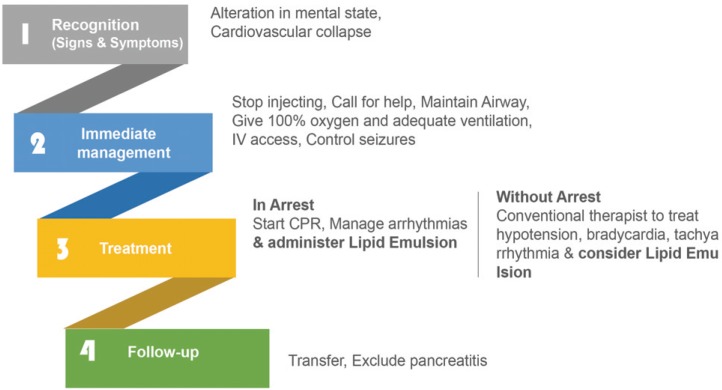
Guidelines presented by AAGBI comprised of four steps: recognition, immediate management, treatment, and follow-up (Fig. 2) [37]. In the first step of recognition, any sudden change in mental status or convulsion should be recognized as symptoms of CNS. For symptoms of CVS, bradycardia, conduction block on the electrocardiogram, or cardiac arrest or lethal arrhythmia should be identified. Because these symptoms could occur immediately after the injection as well as 12 hours later, continued monitoring is necessary. In the second step of immediate management, local anesthetics injection must be stopped as soon as the symptoms of LAST are identified, help must be requested, and intubation should be considered if needed to maintain the airway. Ventilation should be maintained with 100% oxygen. If acidosis occurs due to the toxicity of local anesthetics, compensating with hyperventilation is helpful. One must gain intravenous access and conduct laboratory investigations to treat the symptoms appropriately. If a seizure is active, benzodiazepine-type drugs (e.g., midazolam) or drugs used for anesthesia or sedation (e.g., thiopental or propofol) must be used in small doses to control the seizures. In the third step, the treatment should be different according to the given situation. In the case of an arrest, CPR should be performed quickly. Although toxicity due to local anesthetics is not permanent, when heart function may not recover for over an hour until the drug effect wears off, then cardiopulmonary bypass can be considered. While performing CPR, it is recommended to quickly prepare and inject lipid emulsion. The suggested dose of lipid emulsion to be injected while performing CPR is as follows: 20% lipid emulsion 1.5 mL/kg injected intravenously with bolus injection for about 1 minute, and 15 mL/kg/h infused continuously. If symptoms do not improve, 1.5 mL/kg bolus injection can be injected in 5-minute increments, and the speed of continuous infusion can be doubled. However, the total dose should not exceed 12 mL/kg. In the case of hypotension or arrhythmia without cardiac arrest, a conventional therapy should be performed. In the fourth step of follow-up, the patient should be transferred to a hospital where follow-up care is possible if the symptoms improve. The patient should be monitored for two days to check for pancreatitis using laboratory investigations for amylase or lipase.
The revisions made in the second update in 2012 [38] and the third update in 2018 [27] by the ASRA from the aforementioned 2010 AAGBI guidelines included that cardiac arrest due to LAST must be treated differently from other arrest situations. Although it is recommended that 1 mg of epinephrine be bolus-injected in other arrest situations, the epinephrine dosage should not exceed 1 µg/kg in an arrest situation caused by LAST. Vasopressin, calcium-channel blockers, beta-blockers, and other local anesthetics should be avoided. Additionally, the dosage of lipid emulsion should be altered. The dosage remained unchanged at 1.5 mL/kg for patients weighing < 70 kg, whereas AAGBI recommended 100 mL of bolus injection for 2–3 minutes if the patient weighed ≥ 70 kg. This effectively means that the maximum dosage of lipid emulsion must not exceed 100 mL. Furthermore, it is important to note that the timing of the injection of lipid emulsion is slightly earlier in the revision. It is recommended that the use of lipid emulsion be considered immediately at the first sign of a serious LAST event.
Rosenblatt et al. [44] reported a case of successful recovery in a patient with cardiac arrest caused by LAST due to bupivacaine and mepivacaine, who was treated by injecting 20% lipid emulsion while performing CPR. Since then, many cases of LAST treated using lipid emulsion as an antidote have been reported [11].
There are several mechanisms of lipid emulsion presented. The “lipid sink” hypothesis, which claims that the drugs with high lipid-solubility are pulled from the tissue into the lipid emulsion, and the “metabolic effect” hypothesis, which claims that lipid emulsion reverses the local anesthetics' inhibition of the fatty acid oxidation in the heart, are two of the most widely accepted hypotheses [4546]. Evidence supporting the lipid sink hypothesis includes the fact that lipid emulsion absorbs more of the drug from the tissue to reverse its effect if the drug has higher lipid solubility, and that bupivacaine, which has higher lipid solubility than other local anesthetics, responds well to lipid emulsion therapy [4547]. Furthermore, the reports that lipid emulsion is effective for treating toxicity caused not only by local anesthetics, but also by propranolol, verapamil, and amitriptyline could be related to the high lipid-solubility of these drugs [4849]. Metabolic effect asserts that the lipid emulsion itself has cardiac contraction-promoting and metabolic effects. Bupivacaine inhibits fatty acid oxidation by suppressing the acylcarnitine exchange that is necessary for fatty oxidation to provide energy to the heart. Intralipid recovers fatty acid oxidation in the heart to recover from cardiac arrest due to toxic concentration [505152]. These results could explain the mechanism of directly treating toxicity caused by local anesthetics, rather than the indirect function of the lipid sink [4653].
Go to : 
Although the cases of LAST caused by dental local anesthesia are rare, it can be a serious problem if appropriate steps are not taken. There are no statistical data on the frequency of LAST in dentistry yet. However, the risk of intravenous injection during the commonly performed inferior alveolar nerve block is relatively high (15.3%), and so is the subsequent risk of LAST due to this [54]. The frequency of the use of high-potency, ester-type local anesthetics like bupivacaine in dentistry is not high. Even among the amide-types, only lidocaine is commonly used, and the potency is not high [3]. The risk of LAST still exists even if amide-type anesthetics are used, and the risk is higher if amide-types cannot be used (e.g., lidocaine allergy).
Dentists who perform block anesthesia multiple times a day must be cognizant of the treatments for LAST in case of its occurrence. Fortunately, significant progress has been made after the introduction of lipid emulsion as an effective treatment for LAST. If a LAST event occur, the dentists should be accurately known about fast recognition, use of lipid emulsion depending on the situation, and follow-up to avoid catastrophic events during dental treatments.
Go to : 
References
1. The Korean Dental Society of Anesthesiology. Dental Anesthesiology. 3rd ed. Seoul: Koonja Publishers;2015. p. 299–328.
2. Shin IW, Sohn JT. Lipid emulsion treatment of systemic toxicity induced by local anesthetics or other drugs. J Korean Med Assoc. 2014; 57:537–544.

3. Moore PA, Hersh EV. Local anesthetics: Pharmacology and toxicity. Dent Clin North Am. 2010; 54:587–599. PMID: 20831923.

4. Ciechanowicz SJ, Patil VK. Intravenous lipid emulsion-rescued at LAST. Br Dent J. 2012; 212:237–241. PMID: 22402543.
5. Dillane D, Finucane BT. Local anesthetic systemic toxicity. Can J Anaesth. 2010; 57:368–380. PMID: 20151342.

6. Drasner K. Local anesthetic systemic toxicity: A historical perspective. Reg Anesth Pain Med. 2010; 35:162–166. PMID: 20216034.
7. Weinberg GL. Treatment of local anesthetic systemic toxicity (LAST). Reg Anesth Pain Med. 2010; 35:188–193. PMID: 20216036.

8. Wolfe JW, Butterworth JF. Local anesthetic systemic toxicity: Update on mechanisms and treatment. Curr Opin Anaesthesiol. 2011; 24:561–566. PMID: 21841477.
9. El-Boghdadly K, Pawa A, Chin KJ. Local anesthetic systemic toxicity: Current perspectives. Local Reg Anesth. 2018; 11:35–44. PMID: 30122981.

10. Sites BD, Taenzer AH, Herrick MD, Gilloon C, Antonakakis J, Richins J, et al. Incidence of local anesthetic systemic toxicity and postoperative neurologic symptoms associated with 12,668 ultrasound-guided nerve blocks. Reg Anesth Pain Med. 2012; 37:478–482. PMID: 22705953.

11. Gitman M, Barrington MJ. Local anesthetic systemic toxicity: A review of recent case reports and registries. Reg Anesth Pain Med. 2018; 43:124–130. PMID: 29303925.
12. Bacon B, Silverton N, Katz M, Heath E, Bull DA, Harig J, et al. Local anesthetic systemic toxicity induced cardiac arrest after topicalization for transesophageal echocardiography and subsequent treatment with extracorporeal cardiopulmonary resuscitation. J Cardiothorac Vasc Anesth. 2019; 33:162–165. PMID: 29525187.

13. Hernandez MA, Boretsky K. Chloroprocaine: Local anesthetic systemic toxicity in a 9-month infant with paravertebral catheter. Paediatr Anaesth. 2016; 26:665–666. PMID: 27089835.

14. Pujari VS, Bhandary AS, Bevinaguddaiah Y, Shivanna S. Successful lipid rescue of local anesthetic systemic toxicity following peribulbar block. Med DY Patil. 2015; 8:807.

15. Eizaga Rebollar R, García Palacios MV, Morales Guerrero J, Torres Morera LM. Lipid rescue in children: The prompt decision. J Clin Anesth. 2016; 32:248–252. PMID: 27290983.

16. Vadi MG, Patel N, Stiegler MP. Local anesthetic systemic toxicity after combined psoas compartment-sciatic nerve block analysis of decision factors and diagnostic delay. Anesthesiology. 2014; 120:987–996. PMID: 24694849.
17. Weiss E, Jolly C, Dumoulin JL, Meftah RB, Blanié P, Laloë PA, et al. Convulsions in 2 patients after bilateral ultrasound-guided transversus abdominis plane blocks for cesarean analgesia. Reg Anesth Pain Med. 2014; 39:248–251. PMID: 24682078.

18. Grigg E, Anderson C, Pankovich M, Martin L, Flack S. Systemic ropivacaine toxicity from a peripheral nerve infusion in a medically complex patient. J Clin Anesth. 2015; 27:338–340. PMID: 25862390.

19. Gurnaney H, Kraemer FW, Maxwell L, Muhly WT, Schleelein L, Ganesh A. Ambulatory continuous peripheral nerve blocks in children and adolescents: A longitudinal 8-year single center study. Anesth Analg. 2014; 118:621–627. PMID: 24413546.
20. Shapiro P, Schroeck H. Seizure after abdominal surgery in an infant receiving a standard-dose postoperative epidural bupivacaine infusion. A A Case Rep. 2016; 6:238–240. PMID: 26825992.

21. Tsang TM, Okullo AT, Field J, Mamo P. Lipid rescue for treatment of delayed systemic ropivacaine toxicity from a continuous thoracic paravertebral block. BMJ Case Rep. 2016; 2016:bcr2016215071.

22. Neal JM, Mulroy MF, Weinberg GL. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity: 2012 version. Reg Anesth Pain Med. 2012; 37:16–18. PMID: 22189574.
23. Zink W, Graf BM. The toxicity of local anesthetics: The place of ropivacaine and levobupivacaine. Curr Opin Anaesthesiol. 2008; 21:645–650. PMID: 18784493.

24. Raman S, Madhulaxmi M, Jeevanandan G. Local anesthetics - A review on toxicity. Drug Invent Today. 2019; 12:463–470.
25. Safety Committee of Japanese Society of Anesthesiologists. Practical guide for the management of systemic toxicity caused by local anesthetics. J Anesth. 2019; 33:1–8. PMID: 30417244.
26. Litz RJ, Roessel T, Heller AR, Stehr SN. Reversal of central nervous system and cardiac toxicity after local anesthetic intoxication by lipid emulsion injection. Anesth Analg. 2008; 106:1575–1577. PMID: 18420880.

27. Neal JM, Woodward CM, Harrison TK. The American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity: 2017 Version. Reg Anesth Pain Med. 2018; 43:150–153. PMID: 29356775.
28. Oksuz G, Urfalioglu A, Sekmen T, Akkececi N, Alpay N, Bilal B. Dentists knowledge of lipid treatment of local anaesthetic systemic toxicity. Niger J Clin Pract. 2018; 21:327–331. PMID: 29519981.
29. Slagt C, Ketelaars R, Swenne M, Jan van. Local anesthetic systemic toxicity (LAST) needs treatment. J Emerg Med. 2019; 56:107–108. PMID: 30342862.

30. Wolfe RC, Spillars A. Local anesthetic systemic toxicity: Reviewing updates from the American Society of Regional Anesthesia and Pain Medicine Practice advisory. J Perianesth Nurs. 2018; 33:1000–1005. PMID: 30449428.

31. El-Boghdadly K, Chin KJ. Local anesthetic systemic toxicity: Continuing professional development. Can J Anaesth. 2016; 63:330–349. PMID: 26830640.

32. Di Gregorio G, Neal JM, Rosenquist RW, Weinberg GL. Clinical presentation of local anesthetic systemic toxicity: A review of published cases, 1979 to 2009. Reg Anesth Pain Med. 2010; 35:181–187. PMID: 20301824.
33. Mulroy MF. Systemic toxicity and cardiotoxicity from local anesthetics: Incidence and preventive measures. Reg Anesth Pain Med. 2002; 27:556–561. PMID: 12430104.

34. Ho AM, Karmakar MK, Ng SK, Wan S, Ng CS, Wong RH, et al. Local anaesthetic toxicity after bilateral thoracic paravertebral block in patients undergoing coronary artery bypass surgery. Anaesth Intensive Care. 2016; 44:615–619. PMID: 27608346.

35. Bern S, Weinberg G. Local anesthetic toxicity and lipid resuscitation in pregnancy. Curr Opin Anaesthesiol. 2011; 24:262–267. PMID: 21494132.

36. Gaïes E, Jebabli N, Lakhal M, Klouz A, Salouage I, Trabelsi S. Delayed convulsion after lidocaine instillation for bronchoscopy. Rev Mal Respir. 2016; 33:388–390. PMID: 26596229.
37. Guideline AS. Management of severe local anesthetic toxicity. London: The Association of Anaesthetists of Great Britain & Ireland;2010.
38. Neal JM, Hsiung RL, Mulroy MF, Halpern BB, Dragnich AD, Slee AE. ASRA checklist improves trainee performance during a simulated episode of local anesthetic systemic toxicity. Reg Anesth Pain Med. 2012; 37:8–15. PMID: 22157743.

39. Neal JM, Woodward CM, Harrison TK. The American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity. Reg Anesth Pain Med. 2018; 43:150–153. PMID: 29356775.

40. Kim CH, Yoon JY. Local anesthetics for dental procedure. J Korean Dent Soc Anesthesiol. 2013; 13:71–79.

41. Rosenberg PH, Veering BT, Urmey WF. Maximum recommended doses of local anesthetics: A multifactorial concept. Reg Anesth Pain Med. 2004; 29:564–575. PMID: 15635516.

42. Weinberg G. Lipid rescue resuscitation from local anaesthetic cardiac toxicity. Toxicol Rev. 2006; 25:139–145. PMID: 17192120.

43. Knudsen K, Suurküla MB, Blomberg S, Sjövall J, Edvardsson N. Central nervous and cardiovascular effects of iv infusions of ropivacaine, bupivacaine and placebo in volunteers. Br J Anaesth. 1997; 78:507–514. PMID: 9175963.

44. Rosenblatt MA, Abel M, Fischer GW, Itzkovich CJ, Eisenkraft JB. Successful use of a 20% lipid emulsion to resuscitate a patient after a presumed bupivacaine-related cardiac arrest. Anesthesiology. 2006; 105:217–218. PMID: 16810015.

45. Weinberg GL. Lipid emulsion infusion resuscitation for local anesthetic and other drug overdose. Anesthesiology. 2012; 117:180–187. PMID: 22627464.
46. Ok SH, Sohn JT, Baik JS, Kim JG, Park SS, Sung HJ, et al. Lipid emulsion reverses levobupivacaine-induced responses in isolated rat aortic vessels. Anesthesiology. 2011; 114:293–301. PMID: 21239969.

48. Ozcan MS, Weinberg G. Update on the use of lipid emulsions in local anesthetic systemic toxicity: A focus on differential efficacy and lipid emulsion as part of advanced cardiac life support. Int Anesthesiol Clin. 2011; 49:91–103. PMID: 21956080.
49. Ozcan MS, Weinberg G. Intravenous lipid emulsion for the treatment of drug toxicity. J Intensive Care Med. 2014; 29:59–70. PMID: 22733724.

50. Fettiplace MR, Ripper R, Lis K, Lin B, Lang J, Zider B, et al. Rapid cardiotonic effects of lipid emulsion infusion. Crit Care Med. 2013; 41:e156–e162. PMID: 23531591.

51. Weinberg GL, Palmer JW, VadeBoncouer TR, Zuechner MB, Edelman G, Hoppel CL. Bupivacaine inhibits acylcarnitine exchange in cardiac mitochondria. Anesthesiology. 2000; 92:523–528. PMID: 10691241.

52. Partownavid P, Umar S, Li J, Rahman S, Eghbali M. Fatty acid oxidation and calcium homeostasis are involved in the rescue of bupivacaine induced cardiotoxicity by lipid emulsion in rats. Crit Care Med. 2012; 40:2431–2437. PMID: 22647409.
53. Ok SH, Park CS, Kim HJ, Lee SH, Choi BH, Eun SY, et al. Effect of two lipid emulsions on reversing high-dose levobupivacaine-induced reduced vasoconstriction in the rat aortas. Cardiovasc Toxicol. 2013; 13:370–380. PMID: 23877627.

54. Taghavi Zenouz A, Ebrahimi H, Mahdipour M, Pourshahidi S, Amini P, Vatankhah M. The incidence of intravascular needle entrance during inferior alveolar nerve block injection. J Dent Res Dent Clin Dent Prospects. 2008; 2:38–41. PMID: 23285329.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download