VTE is a potentially fatal complication of hip or knee arthroplasty. The mechanical methods for VTE prophylaxis after hip and knee arthroplasties include mobilization, compression stockings, intermittent pneumatic compression device. In chemical methods for VTE prophylaxis, subcutaneous low-molecular weight heparin (LMWH) has been used for thromboprophylaxis, though other oral chemical agents, such as aspirin are also used. LMWH is applied subcutaneously, and thus, compliance might be an issue as compared with simple oral chemical methods
12). Other oral chemical agents (e.g., dabigatran etexilate [a new direct thrombin inhibitor]) are also available, however, Wolowacz et al.
13) reported this agent is not superior to other known oral chemical agents, especially when it is administered alone to patients. More recently, NOACs (new oral anticoagulants) have been shown to be effective for thromboprophylaxis after total hip arthroplasty (THA) and total knee arthroplasty in randomized controlled trials. Other studies have reported that prophylaxis with RXB for 2 weeks further reduces the risk of VTE following hip or knee arthroplasty
14). The use of RXB is safe, without a statistically significant increase in severe hemorrhage or any bleeding
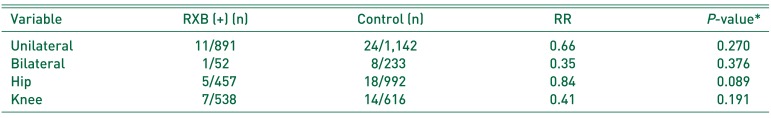
15). In the present study, VTE occurred in 5 of 457 patients (1.09%) administered RXB after hip surgery and in 7 of 538 (1.30%) after knee surgery, which have a decisive effect on more nonspecific lower-extremity symptoms after knee surgery. Kim
16) recommended that all patients undergoing THA are assessed preoperatively for risk of VTE and bleeding to choose appropriate VTE prophylaxis. Also, all patients are recommended to ambulate as early as possible postoperatively to reduce the risk of VTE. Vague and uncertain discomfort in lower legs after knee surgery tend to be evaluated more rigorously after knee surgery than after hip surgery
17), though those that undergo knee arthroplasty may be more susceptible to VTE after surgery due to tourniquet application during surgery
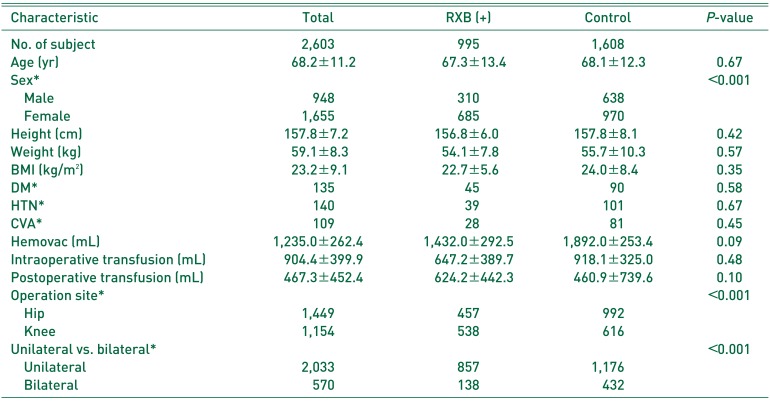
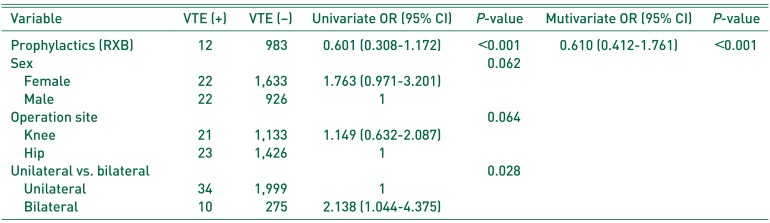
18). In fact, 44 patients diagnosed with VTE within 6 weeks of surgery all underwent knee or hip surgery. Furthermore, the incidence of VTE was reduced by RXB thromboprophylaxis. However, the incidence of PE was higher in the RXB group compared with controls, which we attribute to statistical underpowering due to the relatively small numbers. Furthermore, the RXB group contained a higher proportion of females, simultaneous bilateral procedures, and knee arthroplasties compared with the control group. Patients in the RXB group could have a lower risk to a VTE after surgery. In addition, demographics were significantly different among the 2 study groups, and thus, we adjusted the multivariate analysis for confounders, such as, sex, operation site, and unilateral versus bilateral procedure. We would expect that a larger-scale study would reveal a lower incidence of PE after RXB prophylaxis. Kakkar et al.
10) recommended the use of RXB for more than 5 weeks after surgery, however, the present study indicates RXB usage until POD 14 is sufficient. As VTE mainly occurs at around POD 5 and is uncommon after POD 14
1920), the use of RXB after POD 14 appears to have little advantage. Furthermore, cost or bleeding problems may arise as a result of RXB overuse. In the present study, we used a compressive stocking until 6 week after surgery, and this might have been enough to prevent post-hospitalization VTE in primary arthroplasty patients. This study is meaningful as it includes hip and knee arthroplasty and simultaneous bilateral procedures. Other articles on the topic have focused on thromboprophylaxis after hip joint arthroplasty
921), and the results obtained for thromboprophylaxis in knee arthroplasty patients in the present study support previous results.
Regarding limitations of the present study, we evaluated patients until 6 weeks after surgery, and thus, VTEs that occurred later were not considered. However, VTEs after 6 weeks postoperatively are uncommon. In addition, we included 2,603 patients in our analysis, a sample size which resulted in inadequate statistical power (0.313), but to have achieved a statistical power of >0.6, more than 20,000 patients were needed, which precluded the recruitment of cases treated by one surgeon at one hospital. Nevertheless, the study did reveal a decreasing tendency of VTE development after RXB use. Further multicenter studies are required to increase sample size. The diagnostic accuracy of ultrasonography for detecting DVT also varies according to the technique used. In general, optimal sensitivity for DVT is achieved by using duplex (proximal sensitivity 96%, distal sensitivity 71%, specificity 94%) or triplex ultrasound (proximal sensitivity 96%, distal sensitivity 75%, specificity 94%)
22). Lastly, our uncontrolled before-and-after study (i.e., conducted before and after the introduction of an intervention) and observed differences in performance are assumed to be due to the intervention. Generally, uncontrolled before-and-after studies should not be used to evaluate the effects of quality improvement interventions and the results of studies using such designs have to be interpreted with great caution
23).





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download