Abstract
Circumportal pancreas (CP) is an unusual pancreatic anomaly occurring in 1.1 to 2.5% of individuals, where there is abnormal fusion of the uncinate process to the main pancreatic body occurring to the left of the portal vein-superior mesenteric vein (PV-SMV) junction. Since it was first described in 1987, there have only been a few reports documented in the literature. We recently encountered 2 such cases. Patient 1 was an 81-year-old man who presented with weight loss. Computed tomography (CT) scan revealed an atrophic pancreas with dilated pancreatic duct and a nodule at the head of pancreas, suspicious for a main-duct intraductal papillary mucinous neoplasm (IPMN) with malignant change. During surgery, we discovered that the uncinate process of the pancreas was completely wrapped around the SMV and fused with the main body, resulting in encasement of the PV-SMV junction. The patient also had a replaced right hepatic artery (RHA). Patient 2 was a 76-year-old man who complained of several weeks of abdominal discomfort. A CT scan showed a dilated common bile duct with a distal mass, worrisome for distal cholangiocarcinoma. Intra-operatively, he was similarly found to have union of the uncinate process of the pancreas with the main body occurring to the left of the PV-SMV confluence, with bilateral anomalous hepatic arteries. We present a brief review of the literature surrounding this condition. Although CP is usually asymptomatic, failure to recognize it may lead to serious consequences.
Circumportal pancreas (CP) is an unusual pancreatic anomaly occurring in 1.1 to 2.5% of individuals, where there is an abnormal fusion of the uncinate process to the main pancreatic body occurring to the left of the portal vein-superior mesenteric vein (PV-SMV) junction, resulting in a complete encasement of the vessels. Since it was first reported in 1987 by Suguira et al, there have only been a few case reports and small case series of this condition. Here we present our recent experience with two such patients with this condition, both of whom were diagnosed intraoperatively.
An 81-year-old Chinese male with a background of diabetes mellitus, hypertension, and hyperlipidaemia presented with a three-month history of loss of weight with no other associated symptoms. Clinical examination was unremarkable, and blood tests such as full blood count, renal panel and liver function test were all normal.
A computed tomography (CT) scan revealed an atrophic pancreas with dilated pancreatic duct up to 3 cm, with a 3.5 cm soft tissue nodule at the head of the pancreas. Ca 19-9 was elevated at 44.4. A presumptive diagnosis of main-duct intraductal papillary mucinous neoplasm (IPMN) with possible malignant change was made, and the patient was counselled for total pancreatectomy with splenectomy.
Intra-operatively, after dividing the gastrocolic ligament we entered the lesser sac to visualise the pancreas. Interestingly, we discovered that the uncinate process of the pancreas was completely wrapped around the superior mesenteric vein (SMV) and fused with the main body of the pancreas, resulting in encasement of the PV-SMV junction (Figs. 1, 2). The patient also had a replaced right hepatic artery (RHA) arising from the superior mesenteric artery (SMA). We proceeded to cautiously dissect out the pancreas, taking care not to injure the vessels. The uncinate process was divided with a linear stapler in order to remove the specimen. A retrospective review of the CT scan showed that the pancreatic tissue was indeed encircling the PV-SMV junction, with a branching of the ducts at the head of pancreas (Fig. 3).
Histopathology confirmed a 4.5 cm IPMN with an associated pT1bN0 0.7 cm invasive poorly differentiated ductal adenocarcinoma with no lymphovascular or perineural invasion. All margins were clear.
A 76-year-old man presented with non-specific abdominal discomfort of several weeks duration. Clinical examination was normal. Initial blood test showed obstructive jaundice with serum bilirubin 40 umol/L, alkaline phosphatase 232 u/L and gamma-glutamyl transferase 120 u/L. A CT scan revealed a dilated common bile duct (CBD) with a soft tissue mass in the distal aspect, but the pancreatic duct was not dilated. The patient was suspected to have a distal cholangiocarcinoma and was counselled for a Whipple procedure.
Intraoperatively, the patient was also found to have a union of the uncinate process of the pancreas with the main body occurring to the left of the splenoportal confluence (Figs. 4, 5, 6). In addition, he had bilateral anomalous hepatic arteries, with a replaced RHA arising from the superior mesenteric artery (SMA) and a replaced left hepatic artery (LHA) arising from the left gastric artery (LGA). The fused uncinate portion of the pancreas was transected and the retroportal cut end was oversewn in order to reduce the risk of POPF.
The final histopathology diagnosed adenocarcinoma of the distal CBD arising from a tubular adenoma with high grade dysplasia, pT3N0. The surgical margins were uninvolved.
Circumportal pancreas (CP) is a rare congenital anomaly of the pancreas where there is an abnormal fusion of the uncinate process to the main pancreatic body occurring to the left of the portal vein-superior mesenteric vein (PV-SMV) junction, resulting in complete encasement of the PV or SMV.1 While this occurs normally in pigs, in humans the incidence ranges from 1.1 to 2.5%.2 It was first reported in 1987 by Suguira et al.3 as hypertrophy of the uncinate process of the pancreas encircling the superior mesenteric vein and artery. Various terms have been used to describe it, including portal annular pancreas,45 periportal annular pancreas, complete pancreatic encasement of the portal vein and superior mesenteric vein running through the pancreas.678 Owing to the rarity of the condition, it has been documented mainly as case reports and small case series.
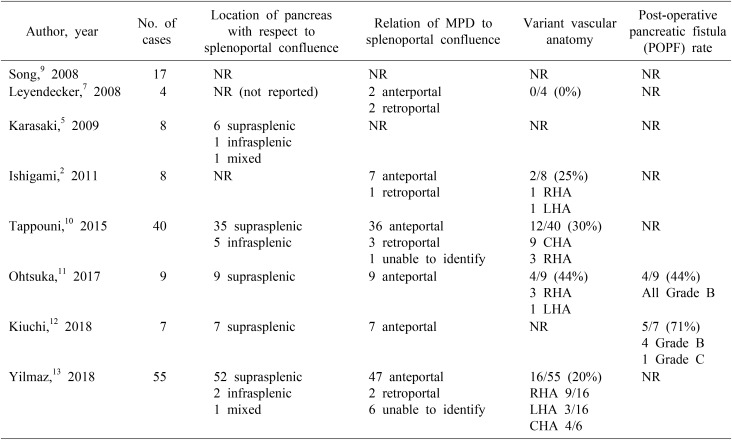
CP occurs due to aberrant fusion of the dorsal and ventral pancreatic primordia, and is a lesser known relative of other anomalies, such as pancreas divisum and annular pancreas.14 Joesph et al.4 proposed a classification based on the location of the fused pancreatic tissue relative to the splenoportal confluence (suprasplenic, infrasplenic or mixed), course of the main pancreatic duct (MPD) with respect to the portal vein (anteportal, retroportal or in association with pancreatic divisum) or by a combination of the two systems.
This condition may be diagnosed by a number of ways. Preoperative CT scans can reveal pancreatic tissue completely encasing the PV and/or SMV. Arterial phase imaging may be useful for demonstrating any variant arterial anatomy. The course of the pancreatic ducts may be better delineated by Magnetic Resonance Cholangiopancreatogram (MRCP), in which a pancreatic duct ring sign has been proposed to be diagnostic,15 or by intraoperative ultrasonography. Most often though, this condition is diagnosed by direct visualization during surgery when the pancreatic tissue is seen to be completely wrapped around the PV-SMV junction.
Although CP usually does not cause any symptoms, it is important for clinicians to be aware of this condition for a number of reasons. First, because of the rarity of the condition, CP may not be easily recognized on pre-operative imaging, as was the case in both of our patients. In the past, it has been mistaken for an extension of the caudate lobe of the liver, periportal lymphadenopathy,16 pancreatic tumour encasing the portal vein817 or even residual neoplasia post resection which resulted in unnecessary surgery.2
Second, CP has been associated with a higher rate of post-operative pancreatic fistula (POPF) following pancreatectomies.15618 This is because division of the pancreatic tissue at the level of the PV-SMV junction in CP results in two cut surfaces (dorsal and ventral to the PV).719 If the retroportal duct is not addressed adequately during surgery, there may be leakage of pancreatic enzymes. In certain instances, the MPD courses posterior to the pancreas. If this is not recognized during a pancreaticoduodenectomy, a pancreaticojejunosotomy (PJ) may be inappropriately created with the minor PD. Techniques to minimize POPF formation in CP include additional pancreatic body resection by dividing the pancreas distal to the fused area resulting in just one cut surface,20 narrowing the pancreatic surface by suturing and division of the region of fused pancreatic tissue,5 and creating a longitudinal side-to-side Puestow PJ if the MPD is significantly dilated.4
Third, in cases such as ours where there is malignant change in a main-duct IPMN, the tumour cells may spread along both the anteportal and retroportal ducts. Failure to identify and completely remove all retroportal pancreatic tissue at the time of surgery may result in R1 resection with cancer cells left behind.
Finally, CP may be associated with variant hepatic arterial anatomy in up to 31% of the cases.11 These include replaced RHA arising from the SMA and replaced LHA arising from the LGA as was the case in both our patients, or even complete encasement of the common hepatic artery in the pancreatic parenchyma.2 If these are not recognized appropriately during surgery and are inadvertently ligated, there may be disastrous consequences.
References
1. Harnoss JM, Harnoss JC, Diener MK, Contin P, Ulrich AB, Büchler MW, et al. Portal annular pancreas: a systematic review of a clinical challenge. Pancreas. 2014; 43:981–986. PMID: 25207658.
2. Ishigami K, Tajima T, Nishie A, Asayama Y, Kakihara D, Nakayama T, et al. The prevalence of circumportal pancreas as shown by multidetector-row computed tomography. Insights Imaging. 2011; 2:409–414. PMID: 22347962.

3. Sugiura Y, Shima S, Yonekawa H, Yoshizumi Y, Ohtsuka H, Ogata T. The hypertrophic uncinate process of the pancreas wrapping the superior mesenteric vein and artery--a case report. Jpn J Surg. 1987; 17:182–185. PMID: 3626212.
4. Joseph P, Raju RS, Vyas FL, Eapen A, Sitaram V. Portal annular pancreas. A rare variant and a new classification. JOP. 2010; 11:453–455. PMID: 20818114.
5. Karasaki H, Mizukami Y, Ishizaki A, Goto J, Yoshikawa D, Kino S, et al. Portal annular pancreas, a notable pancreatic malformation: frequency, morphology, and implications for pancreatic surgery. Surgery. 2009; 146:515–518. PMID: 19715809.

6. Marjanovic G, Obermaier R, Benz S, Bley T, Juettner E, Hopt UT, et al. Complete pancreatic encasement of the portal vein--surgical implications of an extremely rare anomaly. Langenbecks Arch Surg. 2007; 392:489–491. PMID: 17221270.

7. Leyendecker JR, Baginski SG. Complete pancreatic encasement of the portal vein (circumportal pancreas): imaging findings and implications of a rare pancreatic anomaly. J Comput Assist Tomogr. 2008; 32:61–64. PMID: 18303289.
8. Yamazaki S, Kaneko T, Fujinaga Y, Kadoya M, Ochi Y, Sasaki K. CT and MRI features of unclassifiable pancreatic anomaly with superior mesenteric vein running through the pancreatic parenchyma. Eur J Radiol Extra. 2005; 54:59–61.

9. Song SY, Oh JY, Kim Y, Cho OK, Koh BH. Complete pancreatic encasement of portal vein: circumportal pancreas. Eur Radiol Suppl. 2008; 18:350.
10. Tappouni R, Perumpillichira J, Sekala M, Hosseinzadeh K, Clark C, Leyendecker J. Circumportal pancreas: imaging findings in 40 patients. Abdom Imaging. 2015; 40:521–530. PMID: 25248793.

11. Ohtsuka T, Mori Y, Ishigami K, Fujimoto T, Miyasaka Y, Nakata K, et al. Clinical significance of circumportal pancreas, a rare congenital anomaly, in pancreatectomy. Am J Surg. 2017; 214:267–272. PMID: 27871680.

12. Kiuchi R, Mizuno T, Okamura Y, Sugiura T, Kanemoto H, Uesaka K. Circumportal pancreas - a hazardous anomaly in pancreatic surgery. HPB (Oxford). 2018; 20:385–391. PMID: 29198420.

13. Yilmaz E, Celik A. Circumportal pancreas: prevalence, subtypes and vascular variations of 55 patients. Surg Radiol Anat. 2018; 40:407–413. PMID: 29380102.

14. Pardiwala KH, Maker AV. Operative management of portal annular pancreas during pancreaticoduodenectomy. Surgery. 2016; 159:972–974. PMID: 26144878.

15. Lath CO, Agrawal DS, Timins ME, Wein MM. Portal annular pancreas: the pancreatic duct ring sign on MRCP. Radiol Case Rep. 2015; 10:42–45. PMID: 26649117.

16. Connelly TM, Sakala M, Tappouni R. Circumportal pancreas: a review of the literature and image findings. Surg Radiol Anat. 2015; 37:431–437. PMID: 25626884.

17. Luu AM, Braumann C, Herzog T, Janot M, Uhl W, Chromik AM. Circumportal pancreas-a must know pancreatic anomaly for the pancreatic surgeon. J Gastrointest Surg. 2017; 21:344–351. PMID: 27826941.

18. Jang JY, Chung YE, Kang CM, Choi SH, Hwang HK, Lee WJ. Two cases of portal annular pancreas. Korean J Gastroenterol. 2012; 60:52–55. PMID: 22832801.

19. Hashimoto Y, Ross AS, Traverso LW. Circumportal pancreas with retroportal main pancreatic duct. Pancreas. 2009; 38:713–715. PMID: 19629006.

20. Muto J, Mano Y, Harada N, Uchiyama H, Yoshizumi T, Taketomi A, et al. Additional resection of the pancreas body prevents postoperative pancreas fistula in patients with portal annular pancreas who undergo pancreaticoduodenectomy. Case Rep Gastroenterol. 2012; 6:131–134. PMID: 22532811.

Fig. 1
Intra-operative photograph of patient 1 showing the uncinate process of the pancreas fused with the main body, encircling the superior mesenteric vein-portal vein junction (with blue vessel loop).

Fig. 2
Resected specimen of patient 1 with staple line indicating where the fused uncinate process was divided. The surgical ruler delineates the path of the superior mesenteric vein and portal vein.
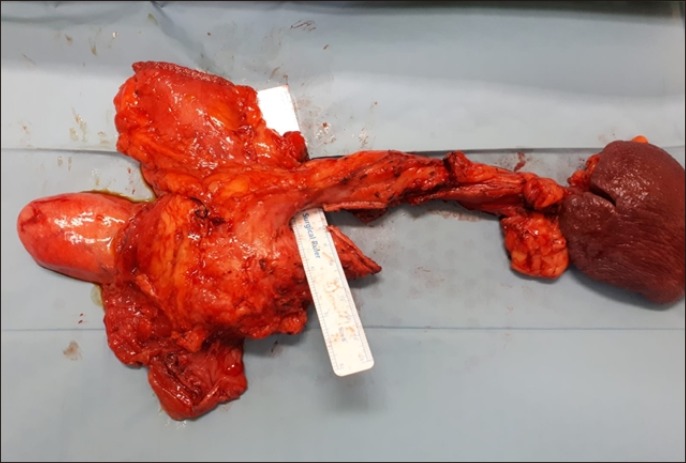
Fig. 3
Computed tomography scan of patient 1 showing the uncinate process fused to the main body (white arrow) and dilated anteportal and retroportal pancreatic ducts.

Fig. 4
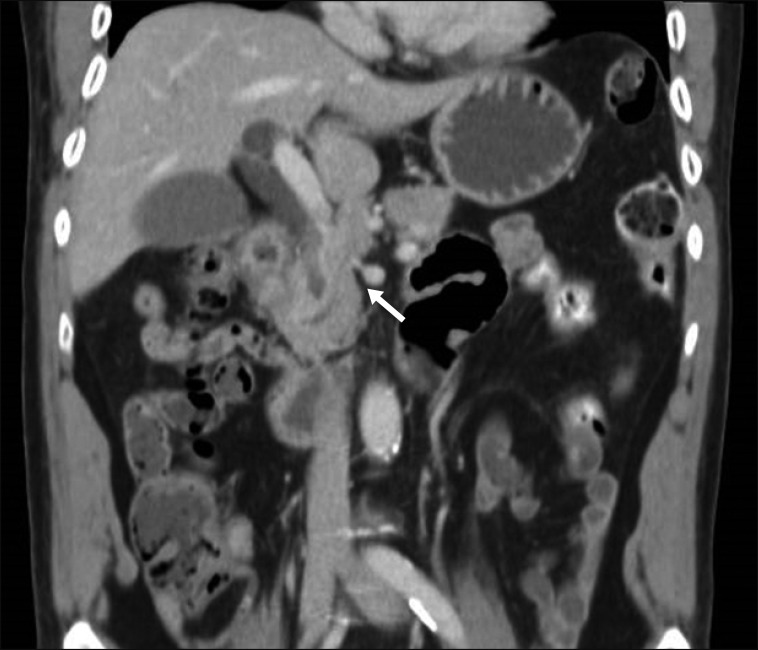
Computed tomography scan of patient 2 showing fused uncinate process fused to the main body, to the left of superior mesenteric vein-portal vein confluence (white arrow).
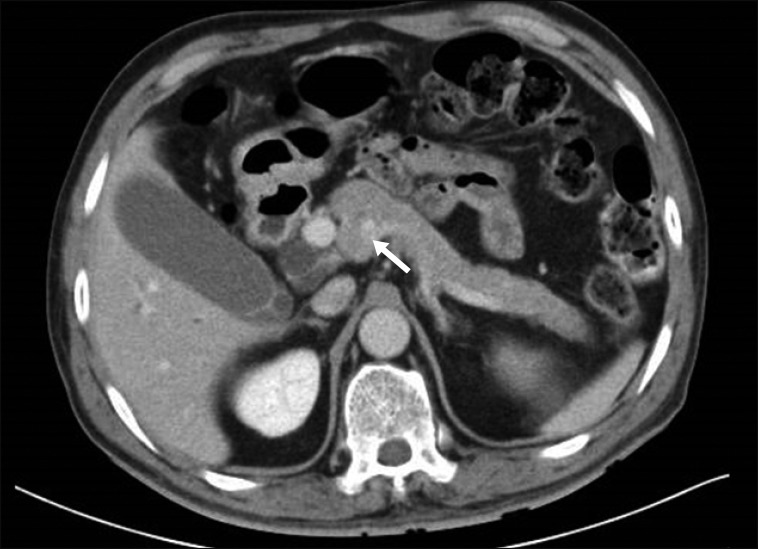
Fig. 5
Computed tomography scan of patient 2 showing fused uncinate process fused to main body, to the left of the superior mesenteric vein-portal vein confluence (white arrow).





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download