This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
Transurethral needle ablation (TUNA) is a minimally invasive procedure for the treatment of symptomatic benign prostatic hyperplasia (BPH). Compared to transurethral resection of the prostate (TURP), office-based TUNA is an attractive alternative as it is minimally invasive and avoids general anaesthesia. The aim of this study is to evaluate the efficacy of single session office-based TUNA.
Materials and Methods
Data of 121 patients who had undergone TUNA was retrieved from June 2008 to March 2017. Patients were followed-up with visits at 1, 3, 6, and 12-months with the International Prostate Symptom Score (IPSS), quality of life (QoL) scoring and uroflowmetry.
Results
Patients were 39 to 85 years old. The prostate volumes were 20.00 to 96.90 mL with a median of 26.95 mL. The median IPSS score pre-TUNA was 19, median QOL score pre-TUNA was 4 and median maximum urinary flow (Qmax) pre-TUNA was 10.3 mL/s. There is 65% improvement of IPSS post-TUNA (p<0.001). There is 75% improvement of QOL post-TUNA QOL (p<0.001). There is 35% improvement of Qmax post-TUNA Qmax (p<0.001). The mean relapse-free survival for TUNA is 6.123 years. The 1st, 3rd, and 5th year relapse-free survival rate were 91.7%, 76.6% and 63.7% respectively.
Conclusions
Our study is the first to investigate the use of a single-setting office-based TUNA requiring minimal sedation in the Asian community. Complication rates were low in our series, with no associated mortality. When applied to selected patients, TUNA is an effective and reasonably safe alternative for the treatment of symptomatic BPH.
Go to :

Keywords: Prostate, Prostatic hyperplasia, Transurethral resection of prostate
INTRODUCTION
Transurethral needle ablation (TUNA) is a minimally invasive procedure for the treatment of symptomatic benign prostatic hyperplasia (BPH) [
1]. It utilises low-level radiofrequency energy to cause selective necrosis of the hyperplastic prostatic tissue whilst preserving the urethra and adjacent structures. Although transurethral resection of the prostate (TURP) remains the gold standard for treatment of BPH, office-based TUNA presents itself as an attractive alternative because of its minimal invasiveness and avoidance of general anaesthesia. TUNA was offered as a single-day office procedure at our centre. We reviewed the outcomes of TUNA performed at our local tertiary unit and affirm its applicability.
Go to :

MATERIALS AND METHODS
This study is approved by National Health group domain specific review board (approval number: NHG DSRB Ref: 2017/01037). Informed consent was waived by the board. Clinico-epidemiological data of 121 patients who had undergone TUNA for symptomatic BPH was retrospectively collected from June 2008 to March 2017. Our selection criteria/indications for patients suitable for TUNA had one or more of the following characteristics: those who had side effects from medical treatment of BPH and unable to tolerate it, those who were not keen for long-term medical therapy, those who failed medical therapy (alpha blocker or 5-alpha reductase or combination drug therapy) due to a lack of benefit or improvement of their lower urinary tract symptoms, and those who were unfit for general anaesthesia.
Patients who were possible candidates for TUNA underwent thorough history taking and examination. Parameters such as the International Prostate Symptom Score (IPSS) and quality of life (QOL) were assessed. Investigations such as urinalysis, measurement of prostate specific antigen (PSA) levels, urine culture, uroflowmetry, bedside ultrasound of the bladder and prostate were performed.
Patients with an abnormal digital rectal examination, or PSA greater than 4.0 ng/mL, had to undergo a trans-rectal ultrasound (TRUS) guided needle biopsy of the prostate to exclude prostate cancer. There is no exclusion criteria for the volume of the prostate but patients with intra-vesical protrusion of the prostate (IPP) of grade III were not offered TUNA. Lastly, patients who had positive urine cultures were treated with antibiotics and had a repeat culture to document clearance prior to TUNA.
All suitable patients were then given rectal suppositories on the night prior to procedure and on the day of procedure and a course of prophylactic antibiotics (ciprofloxacin 500 mg twice a day for one week or augmentin three times a day for one week if patient had ciprofloxacin allergy or previous urine culture is resistance to ciprofloxacin). On the procedural day, patients were given 160 mg of intramuscular gentamicin. A flexible cystoscopy was performed prior to TUNA to exclude high bladder neck, bladder cancer and urethral stricture. Patients were then given 7.5 mg of oral midazolam. TRUS was performed to measure the prostatic volume and maximal transverse length under a periprostatic nerve block using 10 mL of 1% lignocaine. The bladder was decompressed with urinary catheter before subsequent instillation of 60 mL of 2% cold lignocaine solution. TUNA was then performed under direct vision by a single trained urologist using the Prostiva® RF Therapy by Urologix Inc. (Medtronic, MN, USA). The length of the needle and the number of lesions were calculated from measurements obtained during TRUS. Eight areas were ablated for all patients–3 at the bladder neck, 2 at mid-zone and 3 at the apex of the prostate.
Patients were asked to rate their discomfort levels during the procedure using a 10-point scale; 0 for no discomfort to 10–the worst pain the patient had ever experienced. All patients were monitored post-procedurally for 4 hours. They returned home with an indwelling catheter, which would subsequently be removed in a few days.
All patients had scheduled follow-up visits at 1 month, 3 months, 6 months, and 12 months with IPSS, and uroflowmetry performed at each visit. After the one year, all patients were given annual follow-up appointments. However, patients were also given the option of no-follow-up/discharge when they reported personal satisfactory levels of symptomatic improvement and/or showed improvement in IPSS and uroflowmetry parameters at any time of the follow-up visit but were advised to come back when symptomatic again.
IBM SPSS Statistics ver. 24.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Patients??demographic and baseline clinical characteristics were analysed descriptively. The Wilcoxon signed-rank test was used to compare the IPSS, QOL and maximum urinary flow (Qmax) scores between baseline and the best result during the follow-up. The Kaplan–Meier method was used to study the time to secondary treatment, which includes any medical therapy or operation, and also i) sub-group analysis of a group of patients whom although initially responded to therapy, could not be maintained on therapy either due to side effects or unwillingness to take medications for a prolonged duration, versus another group of patients who did not show response to medical therapy at all and ii) subgroup analysis patients who underwent TUNA based on prostate volume. Log-rank test using Bonferroni adjustment was used for all pairwise comparisons, while Cox regression was used for the multivariate comparisons. The Cox proportional hazard regression was used for the multivariate analysis.
Go to :

RESULTS
1. Demographics of the patients
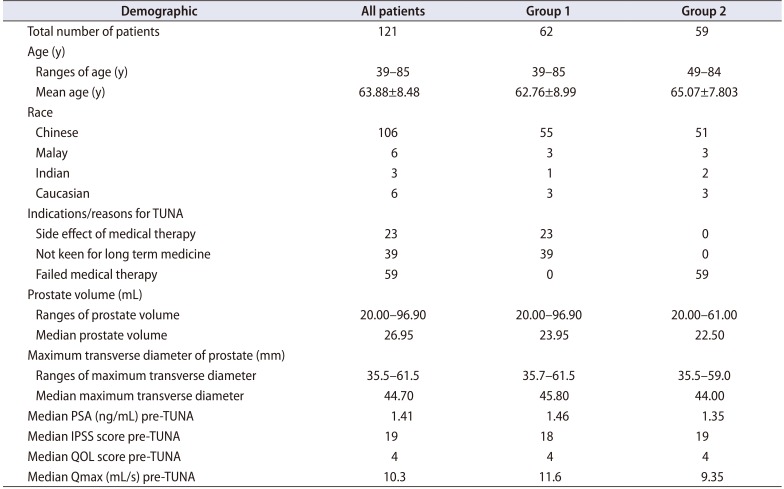
A total of 121 patients underwent TUNA from June 2008 to March 2017. The demographics of the patients are summarized (
Table 1). The age of patients ranged from 39 to 85 years old, with majority (106, 87.6%) were Chinese. During the follow-up, 3 patients died from unrelated causes, 2 cerebrovascular events and 1 cardiovascular event. Of those who underwent TUNA, 23 (19.0%) patients had side effects from medical therapy, 39 (32.2%) were not keen for long term medical therapy and 59 (48.8%) failed medical therapy.
Table 1
Demographic of the patients and the subgroups
|
Demographic |
All patients |
Group 1 |
Group 2 |
|
Total number of patients |
121 |
62 |
59 |
|
Age (y) |
|
|
|
|
Ranges of age (y) |
39–85 |
39–85 |
49–84 |
|
Mean age (y) |
63.88±8.48 |
62.76±8.99 |
65.07±7.803 |
|
Race |
|
|
|
|
Chinese |
106 |
55 |
51 |
|
Malay |
6 |
3 |
3 |
|
Indian |
3 |
1 |
2 |
|
Caucasian |
6 |
3 |
3 |
|
Indications/reasons for TUNA |
|
|
|
|
Side effect of medical therapy |
23 |
23 |
0 |
|
Not keen for long term medicine |
39 |
39 |
0 |
|
Failed medical therapy |
59 |
0 |
59 |
|
Prostate volume (mL) |
|
|
|
|
Ranges of prostate volume |
20.00–96.90 |
20.00–96.90 |
20.00–61.00 |
|
Median prostate volume |
26.95 |
23.95 |
22.50 |
|
Maximum transverse diameter of prostate (mm) |
|
|
|
|
Ranges of maximum transverse diameter |
35.5–61.5 |
35.7–61.5 |
35.5–59.0 |
|
Median maximum transverse diameter |
44.70 |
45.80 |
44.00 |
|
Median PSA (ng/mL) pre-TUNA |
1.41 |
1.46 |
1.35 |
|
Median IPSS score pre-TUNA |
19 |
18 |
19 |
|
Median QOL score pre-TUNA |
4 |
4 |
4 |
|
Median Qmax (mL/s) pre-TUNA |
10.3 |
11.6 |
9.35 |

The prostate volume of the 121 patients ranged from 20.00 to 96.90 mL with a median of 26.95 mL. The maximum transverse diameter of the prostates ranged from 35.5 to 61.5 mm with a median of 44.70 mm. The PSA of patients pre- TUNA ranged from 0.16 to 22.79 ng/mL with a median of 1.41 ng/mL.
The median IPSS score pre-TUNA was 19. The median QOL score pre-TUNA was 4. The Qmax pre-TUNA had a median of 10.3 mL/s.
2. The procedure
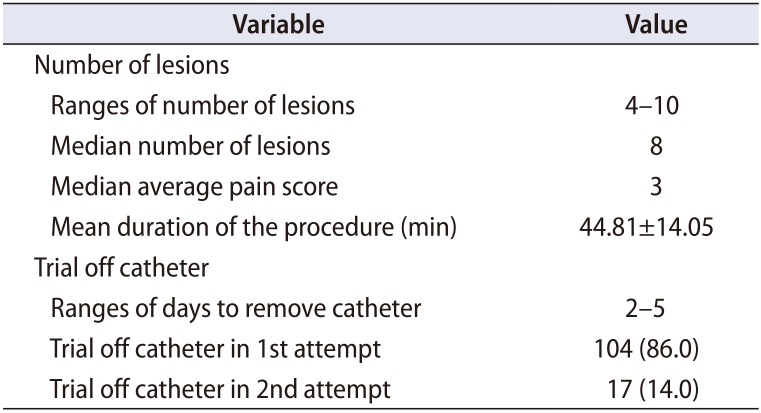
A summary of the procedure is as follows (
Table 2). The median averaged pain score was 3. No patient required cessation of procedure due to pain. The mean duration of the procedure, which was calculated from the start of the flexible cystoscopy, followed by TRUS, to the end of the TUNA was 44.81±14.05 minutes.
Table 2
The procedure outcome
|
Variable |
Value |
|
Number of lesions |
|
|
Ranges of number of lesions |
4–10 |
|
Median number of lesions |
8 |
|
Median average pain score |
3 |
|
Mean duration of the procedure (min) |
44.81±14.05 |
|
Trial off catheter |
|
|
Ranges of days to remove catheter |
2–5 |
|
Trial off catheter in 1st attempt |
104 (86.0) |
|
Trial off catheter in 2nd attempt |
17 (14.0) |

The urinary catheter was removed between 2 to 5 days. Out of 121 patients, 104 (86.0%) could trial off catheter in the 1st attempt which is between two to five days post-TUNA and the rest of 17 patients in the 2nd attempt which is five to seven days after the 1st attempt.
All the procedures were uneventful.
3. IPSS, QOL and Qmax post-TUNA
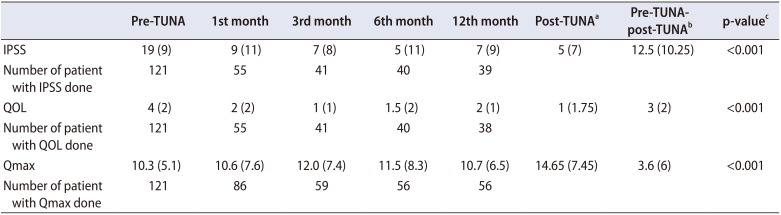
The median IPSS score post-TUNA (
Table 3) on the 1st, 3rd, 6th, and 12th months are as follows: 9, 7, 5, and 7. The median QOL score post-TUNA on the 1st, 3rd, 6th, and 12th months are as follows: 2, 1, 1.5, and 2. The median Qmax score post-TUNA on the 1st, 3rd, 6th, and 12th months are as follows: 10.6, 12.0, 11.5, 10.7.
Table 3
Comparison of the baseline, 1st, 3rd, 6th, and 12 months of IPSS, QOL, and Qmax
|
Pre-TUNA |
1st month |
3rd month |
6th month |
12th month |
Post-TUNAa
|
Pre-TUNA-post-TUNAb
|
p-valuec
|
|
IPSS |
19 (9) |
9 (11) |
7 (8) |
5 (11) |
7 (9) |
5 (7) |
12.5 (10.25) |
<0.001 |
|
Number of patient with IPSS done |
121 |
55 |
41 |
40 |
39 |
|
|
|
|
QOL |
4 (2) |
2 (2) |
1 (1) |
1.5 (2) |
2 (1) |
1 (1.75) |
3 (2) |
<0.001 |
|
Number of patient with QOL done |
121 |
55 |
41 |
40 |
38 |
|
|
|
|
Qmax |
10.3 (5.1) |
10.6 (7.6) |
12.0 (7.4) |
11.5 (8.3) |
10.7 (6.5) |
14.65 (7.45) |
3.6 (6) |
<0.001 |
|
Number of patient with Qmax done |
121 |
86 |
59 |
56 |
56 |
|
|
|

Patients were given the option of no-follow-up/discharge when they reported personal satisfactory levels of symptomatic improvement and/or showed improvement in IPSS and uroflowmetry parameters. Hence, we compared the baseline IPSS with the last recorded post-TUNA IPSS, the baseline QOL with the last recorded post-TUNA QOL, the baseline Qmax with the last recorded TUNA Qmax and the baseline residual urine (RU) with the last recorded post-TUNA RU (
Table 3). The median post-TUNA IPSS is 5. There is 65% improvement of IPSS when baseline was compared to the post-TUNA IPSS (p<0.001). The median best QOL is 1. There is 75% improvement of QOL when baseline was compared to the post-TUNA QOL (p<0.001). The median best Qmax is 14.65. There is 35% improvement of Qmax when baseline was compared to the post-TUNA Qmax (p<0.001).
4. Patients who needed to restart medical therapy and/or needed for additional operation or procedure like TUNA or TURP
In total, 43 out of the 121 patients needed to restart medical therapy, undergo repeat TUNA or was on to have TURP. The mean relapse-free survival time for TUNA is 6.123 years (95% confidence interval [CI], 5.513–6.732). The 1st, 3rd, and 5th year relapse-free survival rate were 91.7%, 76.6%, and 63.7% respectively (
Fig. 1A).
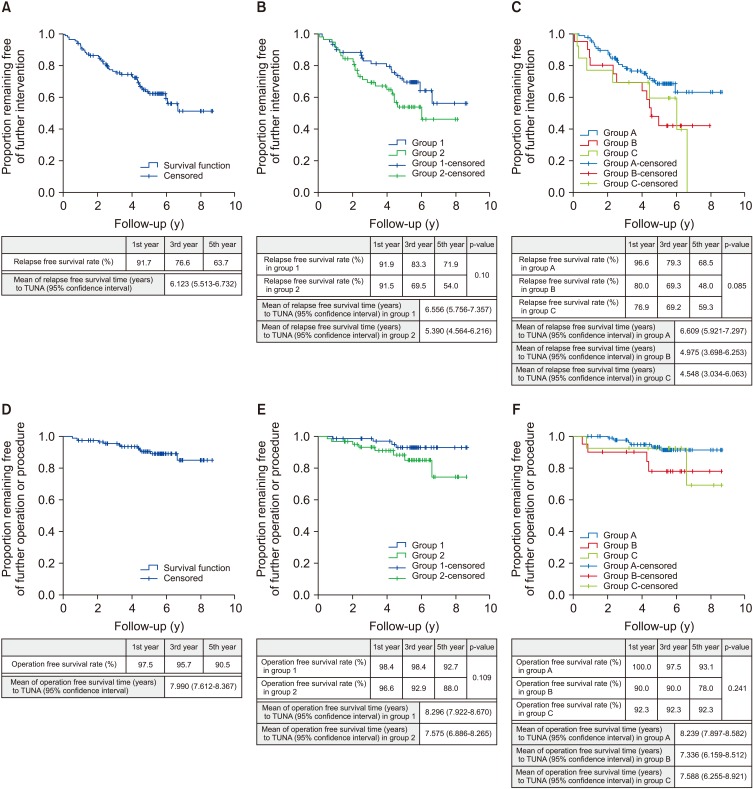 | Fig. 1Kaplan–Meier plots. (A) Kaplan–Meier plot illustrates relapse-free survival rate to transurethral needle ablation (TUNA). (B) Kaplan–Meier plot illustrates relapse-free survival rate to TUNA comparison between group 1: side effects of medical therapy and not keen for long-term medical therapy versus group 2: failed medical therapy. (C) Kaplan–Meier plot illustrates relapse-free survival rate to TUNA comparison between group A: 20.00 to 30.00 mL prostate volume versus group B: 30.01 to 40.00 mL prostate volume versus group C: >40.01 mL prostate volume. (D) Kaplan–Meier plot illustrates operation free survival rate to TUNA. (E) Kaplan–Meier plot illustrates operation free survival rate to TUNA comparison between group 1: side effects of medical therapy and not keen for long-term medical therapy versus group 2: failed medical therapy. (F) Kaplan–Meier plot illustrates operation free survival rate to TUNA comparison between group A: 20.00 to 30.00 mL prostate volume versus group B: 30.01 to 40.00 mL prostate volume versus group C: >40.01 mL prostate volume.
|
We divided patients who underwent TUNA into two groups. The first group (group 1: 62 patients) was patients who underwent TUNA due to side effects from medication, or whom were not keen on taking long term medication. The second group (group 2: 59 patients) were patients who had failed medical therapy. A comparison of the 2 subgroups is demonstrated in
Table 1. The mean relapse-free survival time was 6.556 years (95% CI, 5.756–7.357) for group 1 and 5.390 years (95% CI, 4.564–6.216) for group 2. The 1st, 3rd, and 5th year relapse-free survival rate for group 1 were as follows: 91.9%, 83.3%, and 71.9%, and for group 2 were as follows: 91.5%, 69.5%, and 54.0% (
Fig. 1B). The results have shown that patients in group 2 have shorter relapse-free time compare to group 1, but the difference was not statistically significant (p=0.10).
We also sub-analysed patients who underwent TUNA based on prostate volume. The first group (group A: 88 patients) were patients with prostate volume of 20.00 to 30.00 mL. The second group (group B: 20 patients) were patients with prostate volume of 30.01 to 40.00 mL. The third group (group C: 13 patients) were patients with prostate volume more than 40.01 mL. The mean relapse-free survival time was 6.609 years (95% CI, 5.921–7.297) for group A, 4.975 years (95% CI, 3.698–6.253) for group B, and 4.548 years (95% CI, 3.034–6.063) for group C. The 1st, 3rd, and 5th years relapse-free survival rate for group A were as follows: 96.6%, 79.3%, and 68.5%, for group B were as follows: 80.0%, 69.3%, and 48.0%, and for group C were as follow 76.9%, 69.2%, and 59.3% (
Fig. 1C). Patients in group C have the shortest relapse-free time then followed by group B, and with group A having the longest relapse-free time, but the difference was not statistically significant (p=0.085). The comparison is also not statistically significant for pairwise comparison, group A versus group B (p=0.150), group A versus C (p=0.372) and group B versus C (p=1.000).
5. Patients who required an additional operation or procedure like TUNA or TURP
Out of 121 patients, 12 needed additional TUNA or TURP. Out of the 12 patients, 1 had repeat TUNA and the rest had TURP. The mean operation free survival time for patient who has undergone TUNA was 7.99 years. The 1st, 3rd, and 5th year operation free survival rate were as follows: 97.5%, 95.7%, and 90.5% (
Fig. 1D).
Again, we sub-divided our patients into group 1 and 2 as per above.
The mean operation free survival time for TUNA is 8.296 years (95% CI, 7.922–8.670) for group 1 and 7.575 years (95% CI, 6.886–8.265) for group 2. The 1st, 3rd, and 5th year operation free survival rate for group 1 were as follows: 98.4%, 98.4%, and 92.7%, and for group 2 were as follows: 96.6%, 92.9%, and 88.0% (
Fig. 1E). Patients in group 2 have a shorter time to re-operation compare to group 1, but the difference was not statistically significant (p=0.109).
The mean operation free survival time for patients who have undergone TUNA was 8.239 years (95% CI, 7.897–8.582) for group A, 7.336 years (95% CI, 6.159–8.512) for group B, and 7.588 years (95% CI, 6.255–8.921) for group C. The 1st, 3rd, and 5th year operation free survival rate for group A were as follows: 100.0%, 97.5%, and 93.1%, for group B: 90.0%, 90.0%, and 78.0%, and for group C: 92.3%, 92.3%, and 92.3% (
Fig. 1F). Patients in group A had a longer time interval to re-operation compared to group B and C, but the difference was not statistically significant (p=0.241). The comparison is also not statistically significant for pairwise comparisons, group A versus group B (p=0.243), group A versus C (p=1.000) and group B versus C (p=1.000).
6. Other adverse effects
Of the 121 patients, 2 developed urethral strictures, 1 had urosepsis requiring hospitalisation and 1 had persistent hematuria requiring blood transfusion and subsequent cysto-diathermy.
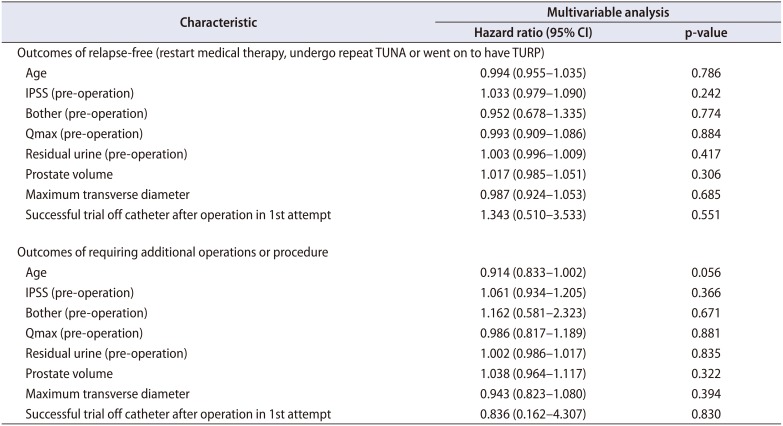
7. Multivariate analysis
Multivariate analysis was performed. The analysis did not reveal any risk factors for recurrence. The analysis outcome is provided in
Table 4.
Table 4 demonstrating that when Cox proportional hazards regression of variables was performed, there were no associations found between the studied variables (age, IPSS, Bother scores etcetera) and the failure of TUNA.
Table 4 demonstrates that after Cox proportional hazards regression of variables, there were no associations between studied variables (as in
Table 4) and the eventual outcomes of requiring additional operations or procedure.
Table 4
Multivariate analysis (Cox proportional hazards regression of variables associated with the outcomes)
|
Characteristic |
Multivariable analysis |
|
Hazard ratio (95% CI) |
p-value |
|
Outcomes of relapse-free (restart medical therapy, undergo repeat TUNA or went on to have TURP) |
|
Age |
0.994 (0.955–1.035) |
0.786 |
|
IPSS (pre-operation) |
1.033 (0.979–1.090) |
0.242 |
|
Bother (pre-operation) |
0.952 (0.678–1.335) |
0.774 |
|
Qmax (pre-operation) |
0.993 (0.909–1.086) |
0.884 |
|
Residual urine (pre-operation) |
1.003 (0.996–1.009) |
0.417 |
|
Prostate volume |
1.017 (0.985–1.051) |
0.306 |
|
Maximum transverse diameter |
0.987 (0.924–1.053) |
0.685 |
|
Successful trial off catheter after operation in 1st attempt |
1.343 (0.510–3.533) |
0.551 |
|
Outcomes of requiring additional operations or procedure |
|
Age |
0.914 (0.833–1.002) |
0.056 |
|
IPSS (pre-operation) |
1.061 (0.934–1.205) |
0.366 |
|
Bother (pre-operation) |
1.162 (0.581–2.323) |
0.671 |
|
Qmax (pre-operation) |
0.986 (0.817–1.189) |
0.881 |
|
Residual urine (pre-operation) |
1.002 (0.986–1.017) |
0.835 |
|
Prostate volume |
1.038 (0.964–1.117) |
0.322 |
|
Maximum transverse diameter |
0.943 (0.823–1.080) |
0.394 |
|
Successful trial off catheter after operation in 1st attempt |
0.836 (0.162–4.307) |
0.830 |

Go to :

DISCUSSION
Our study represents the first single-setting office-based TUNA requiring minimal sedation done in the Asian community. TUNA is able to significantly improve IPSS, QOL and Qmax. The complication rates for TUNA were low in our series, with no associated mortality and only 2 of 121 (1.7%) requiring hospitalisation post procedurally. When applied to selected patients, TUNA is an effective and reasonably safe alternative for the treatment of symptomatic BPH.
In a study performed by Leocádio et al. [
2], the methods of anaesthesia were similar to ours. However, no periprostatic administration of lignocaine was performed. This study reported a mean pain score of 4.8 compared to 3 in our study. This suggests that the additional use of a periprostatic nerve block with lignocaine may play a significant role in pain relief.
In our study, TUNA significantly improved BPH parameters (65% improvement of IPSS, 75% improvement of QOL and 35% improvement in Qmax) post-TUNA when compared to pre-TUNA values. Our results corroborate the findings of Bouza et al. [
3], which further affirms the efficacy of officebased TUNA .
In our study, the median time to symptom recurrence was 6.123 years. In comparison, a 2007 study by Rosario et al. [
4] reported a median time to symptom recurrence of 20 months. Although this study demonstrates a higher efficacy of TUNA compared to Rosario et al. [
4], this may be confounded by the fact 100% of their recruited patients had failed initial medical therapy, whereas in our study, only group 2 had failed medical therapy.
Even in group 2, 54.0% managed to remain symptom-free by the 5th year of follow-up and the median time to symptom recurrence was 5.390. This demonstrates a higher efficacy of TUNA in prolonging median time to symptom recurrence, and establishing long-lasting symptom-free intervals. In a meta-analysis on TUNA by Bouza et al. [
3], only 237 of 1,036 patients treated with TUNA required medical and/or surgical treatment there after amounting to a rate of only 19.07%. There was otherwise no mention of the period of follow-up and the median time to symptom recurrence. Our study showed that only 9.5% of the patients required additional procedure or operation post 5th years of treatment. This result is comparatively better than those reflected by Bouza et al. [
3] which showed that 186 of 1,036 patients (18.0%) eventually required surgical interventions, although the follow-up interval was not clearly stated in this meta-analysis. Our study also proposed good efficacy of TUNA compared to the study by Rosario et al. [
4], the latter demonstrating that 48.5% of patients required eventual surgical intervention.
Although our study did not identify factors for treatment failure, we excluded patients with grade III IPP. This may account for our high success rate compared to Rosario et al. [
4]. Wang et al. [
5] which suggested that patients with IPP grade III with small prostates would be achieve better treatment outcomes with TURP. Purposeful exclusion of patients with IPP grade III would improve the overall effectiveness of TUNA, particularly in terms of achieving long-lasting control of symptoms.
In terms of cost benefits, the cost of TUNA in our institution is estimated to be United States dollar (USD) $3,600 and the cost of combination medical treatment is estimated to be USD$1,080 per year, with an estimate of 3 years and 4 months to reach breakeven. The mean time to symptom recurrence of 6.123 years in our study, demonstrating cost effectiveness to perform the procedure.
Although not statistically significant in our series, TUNA seemed to be beneficial for patients who chose to have the procedure due to intolerance to medication or reluctance for chronic medications. It may be assumed that these patients had a milder form of BPH compared to those who had already failed medical therapy (group 2), and hence naturally performed better after TUNA. This author feels that TUNA is a feasible alternative to patients with symptomatic BPH who are intolerant to the side effects of alpha-blockers. TUNA may also be offered to those who have failed medical therapy, although more in-depth evaluation is advised (prostate size, IPP) to exclude those that may benefit more from upfront TURP. TUNA was seen to be beneficial for patients with smaller prostate volume especially for prostate volumes ranging from 20.00 to 30.00 mL (although this was not statistically significant). As TUNA can be done without general anaesthesia, TUNA will benefit patients who have high risk of general anaesthesia and have a small prostate volume of 20 to 30 mL with symptomatic BPH. The limitations of our study include a small sample size, and not being able to trace patients who had a deterioration of BPH symptoms after being discharged and had not sought treatment from public hospitals. This could have led to an over-estimation of our relapse-free rate.
Go to :

CONCLUSIONS
This study is the first single-day office TUNA study done in an Asian community. Our study has shown that TUNA is a safe and effective procedure with patients with symptomatic BPH. It is suitable for those cannot tolerate the side effects of medical therapy or those whom are not keen for long term medical therapy, high risk of general anaesthesia and/or have a small prostate volume of 20 to 30 mL.
Go to :










 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download