Abstract
Purpose
Many studies have concluded that cancer patients may have better outcomes when their surgery is performed in high-volume centers, especially when the procedure is pancreaticoduodenectomy (PD). However, some studies concluded that experienced surgeons or incorporation of expertise from high-volume centers may achieve satisfactory outcomes after PD in low-volume centers.
Methods
I retrospectively collected and analyzed the outcomes of PD for periampullary cancers treated with curative intent in my center.
Results
From August 2, 2005 to December 10, 2018, 160 pancreatic resections were done with curative intent in my center. The number of operations per year was 1 in 2005 and gradually increased to 21 in 2018. Thirty-day mortality was 0, and 90-day mortality was 1 (0.6%). Morbidity was found in 65 cases (40.6%). The median follow-up period was 23.2 months and 5-year survival rates were 28.5% for pancreas head cancer, 48.2% for distal CBD cancer, and 72.6% for AOV cancer. I divided patients into 2 groups by the number of annual operations, which is more than 21 per 2 years. The 2 groups showed no differences in terms of morbidity and mortality.
Many studies have concluded that cancer patients may have better outcomes with respect to morbidity, mortality, and survival rate when their surgery is performed in high-volume centers than in low-volume centers. And when this concern is focused on performing pancreaticoduodenectomy (PD), which is thought to be a high-risk cancer surgery, the relationship between volume and outcome invariably increased [123456]. However, some studies concluded that experienced surgeons or incorporation of expertise from high-volume centers may achieve satisfactory outcomes after PD in low-volume centers [789].
My center opened on August 1, 2005 and the volume of PD gradually increased from 1, which was my first PD, to 21 per year, and performed by one surgeon. The Department of Surgery in my center is subdivided into several specialized divisions and has a Hepato-Pancreato-Biliary unit with specialists who are well trained from high-volume centers. Although high volume would influence the outcome of PD, the volume might not be the main or only reason influencing the outcome. I hypothesize that comparatively good outcome could be attained in a low-volume center if well organized and well prepared. I assessed the outcomes of PD for periampullary cancers in my center.
Data were retrospectively collected from August 2005 to December 2018. A total of 209 operations were undergone from August 2, 2005 to December 10, 2018 for periampullary tumors. The diagnoses and the name of these operations are listed in Tables 1 and 2. Among the 209 operations, 165 operations were done for periampullary cancers, and 160 operations were done with curative intent. Analysis of these 160 operations was carried out. Demographic data, data from the operation, postoperative morbidity, and postoperative mortality were described. Oncologic outcomes including TNM staging according to American Joint Committee on Cancer (AJCC) 8th edition, cumulative survival rates according to the type of periampullary cancers were analyzed. All analyzes were performed by using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Survival curves were estimated by the Kaplan-Meier method. A P-value <0.05 was considered statistically significant. I divided patients into 2 groups by operation date. A group is from 2005 to 2012 and B group is from 2013 to 2018. The reasoning is that the cut value for the appropriate volume for major pancreatic resection from the Health Insurance Review & Assessment Service in Korea is more than 21 per 2 years. Comparisons between the 2 groups were performed using chi-square test, independent samples t-test. A P-value <0.05 was considered statistically significant.
This study was approved by the Institutional Review Board of Konkuk University Medical Center (approval number: KUMC 2019-03-025).
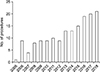
A total of 160 pancreatic resections for periampullary cancers with curative intent were performed for 13 and a half years. The number of operations per year was 1 in 2005, which was the first PD done by me, and it gradually increased to 21 in 2018. This is shown in Fig. 1. The mean patient age was 64.3 years ranging from 35 to 84 years. The male patient was 100 and the female was 60. Pancreatic head cancer was 60 cases (37.5%), distal common bile duct (CBD) cancer was 58 cases (36.25%), ampulla of Vater (AOV) cancer was 38 cases (23.75%), and duodenal cancer was 4 cases (2.5%). The types of operation were pylorus preserving pancreaticoduodenectomy (PPPD), Whipple's procedure, and total pancreatectomy, and each number of times is listed in Table 3. Segmental resection or wedge resection of the right lateral margin of the portal vein or superior mesenteric vein was needed in 9 cases (5.6% of all, 15% of pancreas head cancer) and these were all pancreas head cancer cases. The resection of the transverse colon was needed in 2 cases and resection of the mesentery of transverse colon was needed in 6 cases; these cases were also pancreas head cancer cases. Additional resections for negative margin were done in 8 cases of pancreas head cancer (13.3%), 9 cases of distal CBD cancer (15.5%) and achieved negative cancer margins. Negative resection margin at the time of surgery turned to positive after surgery in 1 distal CBD cancer case, and this patient was put on combined radiotherapy and chemotherapy postoperatively. The procedure was converted from PPPD to total pancreatectomy in 1 pancreas head cancer case due to severe dysplasia of margin, another conversion was done in an AOV case due to a combined lesion of intraductal papillary mucinous neoplasm, which showed severe dysplasia. Neoadjuvant chemotherapy was done in 1 distal CBD cancer case (1.7%) and 4 pancreas head cases (6.7%). Mean operative time was 413 minutes ranging from 285 to 645 minutes. The mean estimated blood loss during the operation was 985.5 mL ranging from minimal to 8,000. Transfusion was done in 59 cases (36.9%). Mean admission days were 23.8 days ranging from 10 to 99 and mean follow-up months were 30.6 months ranging from 49 days to 162.2 months. These patient characteristics and procedures are summarized in Table 3. Adjuvant treatment, which usually was chemotherapy and sometimes was chemoradiotherapy, was done in 47 cases (78.3%) for pancreas head cancer, in 35 (60.3%) for distal CBD cancer, in 15 (39.5%) for AOV cancer, and in 2 (50.0%) for duodenal cancer.
Outcomes of pathologic staging according to AJCC 8th edition is listed in Table 4. Thirty-day mortality was 0, and 90-day mortality was 1 (0.6%, overall) of distal CBD cancer. Morbidity was found in 65 cases (40.6%). Postoperative pancreatic fistula was found in 36 cases (22.5%) with 20 (12.5%) grade Bs and no grade C. Intraabdominal abscess was found in 14 cases (8.8%), delayed gastric emptying in 12 (7.5%), postoperative bleeding in 6 (3.8%) with 3 (1.9%) delayed bleeding cases. Other complications are listed in Table 4. The median follow-up period for 160 periampullary cancers was 23.2 months and 5-year survival rates were 28.5% for pancreas head cancer, 48.2% for distal CBD cancer, 72.6% for AOV cancer, and no calculable result for duodenal cancers. The survival curve for each type of periampullary cancers is in Fig. 2. The type was a significant univariate factor for survival (P = 0.001).
When I divided patients into 2 groups according to the time of operation to compare outcomes of early group and late group, age, sex ratio, type of periampullary cancers, type of operation, and operation time were not statistically different in groups A and B. However, there was a tendency for the proportion of pancreas head cancer to increase and the proportion of AOV cancer decrease. The only meaningful and statistical decrease was in admission days and this is summarized in Table 5. Morbidity and mortality were not different between the 2 groups except 1 and this is summarized in Table 6.
Although PD is a high-risk surgical procedure, many studies have reported that better operative results, expressed by perioperative mortality below 5%, have been obtained by several centers and surgeons with a high volume of PDs. [610111213] The appropriate number of cases to define a center and a surgeon as high volume in PDs differs among several studies. Some studies determined high-volume centers as those with more than 20 operations per year and high-volume surgeons as those performing more than 11 operations per year [10111213]. Determining factors in several studies for better outcomes in mortality after PD were the high volume as first and the experience of a well-trained surgeon as secondary [101112131415]. The cut value for the appropriate volume for the major pancreatic resection from the Health Insurance Review & Assessment Service in Korea is more than 21 per 2 years.
In my study, one surgeon underwent all procedures. Therefore, an appropriate number for high volume was achieved for a surgeon for the year of 2013. However, for a center, it was achieved from the year of 2017 [10111213]. According to the cut value from the Health Insurance Review & Assessment Service in Korea, my center became qualified from the year of 2013. Therefore, I analyzed whole cases and divided them into 2 groups, group A from 2006 to 2012, group B from 2013 to 2018, and compared outcomes of the 2 groups. A 30-day mortality rate of 0%, 90-day mortality 0.6%, and a morbidity rate of 40.6% are comparable to those within the standards of excellence obtained by high-volume PD centers [71617181920]. One mortality was the case of a 73-year-old male with distal CBD cancer. He had a small intraabdominal abscess and managed with antibiotics. However, this approach failed and the small abscess progressed to multiple larger abscesses along the operative field. A percutaneous drainage (PCD) insertion was done on postoperative day 18 to control the abscess without good drainage. Multiple PCD was done thereafter, but to no avail. The patient died of multi-organ failure on postoperative day 51. As occurs with high-volume centers, pancreatic fistula, intraabdominal abscess, and delayed gastric emptying were the most frequent complications. Operation time appears to be a little longer compared to other studies. My data was collected from anesthesia records, and the operation time starts not from the moment of incision but from the moment of starting skin preparation, and ends with the dressing of the wound. Also, every year, I retain aid during the operation from a new third year or fourth-year resident, not from a fellow who would be far better helpful. This might be the reason for the slightly longer operation time and uneven operation time.
The median follow-up period for 160 periampullary cancers was 23.2 months and 5-year survival rates were 28.5% for pancreas head cancer, 48.2% for distal CBD cancer, 72.6% for AOV cancer, and no calculable result for duodenal cancers. To compare the survival outcome strictly, we need to separate the type and stage of periampullary cancers though the small numbers in the categories of periampullary malignancy negate any useful comments regarding their survivorship. Yet, these outcomes from my center are not different from other studies. [61819202122] Thus, also at the aspect of survival, small volume centers' outcomes might not be inferior to those of high-volume centers.
When compared, groups A and B as shown in Tables 5 and 6, regarding age, sex ratio, type of periampullary cancers, type of operation, and operation time were not statistically different, though there was a tendency for the proportion of pancreas head cancer to increase and the proportion of AOV cancer decrease. In the beginning of the center, many pancreatic head cancer patients went to other well-known centers in Seoul for surgery, but as time passed, the proportion of those patients decreased. Operation time was not much reduced against my expectations, and estimated blood loss even increased statistically. This may have been due to the increase of pancreatic head cancer patients, and indeed, segmental resection or wedge resection of the right lateral margin of the portal vein or superior mesenteric vein being done in only group B. The only meaningful and statistical decrease was in admission days. Morbidity and mortality were not statistically different between the 2 groups except in the number of hepatic artery embolization. I tried to compare survival rates between the 2 groups for each disease; P-value of overall survival rates for pancreatic head cancer was 0.050, for distal CBD cancer was 0.867, and for AOV cancer was 0.223. However, due to the small numbers, different median follow-up periods, and different staging in the categories of periampullary malignancy of the 2 groups, the results would not be reliable. One explanation is that there were only 2 out of 17 (11.8%) stage I cases in group A, but 13 out of 43 (30.2%) stage I cases in group B in pancreas head cancer. To adjust the staging between the 2 groups, more cases are needed for each group, and in this study, survival rates could not be comparable in the 2 groups for this reason. Some insisted experience and technical expertise as being the most important factors in achieving good results after PD, and others add more factors besides experience which are an optimized hospital structure and a well-equipped center [2122232425]. And in my study, small volume or early period did not influence the outcome between the 2 groups. When I was a resident (4 years) or a fellow (1 year), about 50 to 70 PDs were performed annually in the center. After moving to my new center, I went abroad as a research fellow for 2 years while my new center was being built. I returned 5 months before the opening of my new center and worked on filling in the blanks of my clinical experiences and on preparing to set up the system for the new center. To that end, I visited the center in which I was trained, to observe and participate in operations such as liver transplants and PDs, with some new residents, and with some new nurses for my new center several times. As a conclusion to these studies, being well trained in a high-volume center as a resident and a fellow, and gradually growing the number of patients in a well-designed center, are the likely factors to good outcome in my center, at least it terms of morbidity and mortality. Also, experiences from other operations in the Hepato-Pancreato-Biliary field would be helpful to good outcome of PDs in my center. I performed a great number of laparoscopic cholecystectomies, major or minor liver resections, distal pancreatectomies, extended cholecystectomies, living donor liver transplants, and deceased donor liver transplants in the same period. The experiences from these surgeries have greatly benefitted me.
In conclusion, comparing outcomes of my center with other centers and comparing outcomes between the 2 groups according to annual numbers demonstrated that the only volume is not the factor in determining the outcome of PD for periampullary cancers. A well-trained low-volume surgeon may perform PD safely at a well-equipped low-volume center, and accumulated experience from other surgeries would lead to good outcomes after PD.
Figures and Tables
 | Fig. 2Survival rates of 160 periampullary cancers according to histologic types. AOV, ampulla of Vater; CBD, common bile duct. |
References
1. Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998; 280:1747–1751.

2. Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003; 349:2117–2127.

3. Finlayson EV, Birkmeyer JD. Effects of hospital volume on life expectancy after selected cancer operations in older adults: a decision analysis. J Am Coll Surg. 2003; 196:410–417.

4. Fong Y, Gonen M, Rubin D, Radzyner M, Brennan MF. Long-term survival is superior after resection for cancer in high-volume centers. Ann Surg. 2005; 242:540–544.

5. Birkmeyer JD, Sun Y, Goldfaden A, Birkmeyer NJ, Stukel TA. Volume and process of care in high-risk cancer surgery. Cancer. 2006; 106:2476–2481.

6. van der Geest LG, van Rijssen LB, Molenaar IQ, de Hingh IH, Groot Koerkamp B, Busch OR, et al. Volume-outcome relationships in pancreatoduodenectomy for cancer. HPB (Oxford). 2016; 18:317–324.

7. Schmidt CM, Turrini O, Parikh P, House MG, Zyromski NJ, Nakeeb A, et al. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: a single-institution experience. Arch Surg. 2010; 145:634–640.
8. Kanhere HA, Trochsler MI, Kanhere MH, Lord AN, Maddern GJ. Pancreaticoduodenectomy: outcomes in a low-volume, specialised Hepato Pancreato Biliary unit. World J Surg. 2014; 38:1484–1490.

9. Chedid AD, Chedid MF, Winkelmann LV, Grezzana Filho TJ, Kruel CD. Achieving good perioperative outcomes after pancreaticoduodenectomy in a low-volume setting: a 25-year experience. Int Surg. 2015; 100:705–711.

10. Sosa JA, Bowman HM, Gordon TA, Bass EB, Yeo CJ, Lillemoe KD, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg. 1998; 228:429–438.

11. Gouma DJ, van Geenen RC, van Gulik TM, de Haan RJ, de Wit LT, Busch OR, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000; 232:786–795.

12. Kotwall CA, Maxwell JG, Brinker CC, Koch GG, Covington DL. National estimates of mortality rates for radical pancreaticoduodenectomy in 25,000 patients. Ann Surg Oncol. 2002; 9:847–854.

13. Rosemurgy A, Cowgill S, Coe B, Thomas A, Al-Saadi S, Goldin S, et al. Frequency with which surgeons undertake pancreaticoduodenectomy continues to determine length of stay, hospital charges, and inhospital mortality. J Gastrointest Surg. 2008; 12:442–449.

14. Ho V, Heslin MJ. Effect of hospital volume and experience on in-hospital mortality for pancreaticoduodenectomy. Ann Surg. 2003; 237:509–514.

15. Topal B, Van de Sande S, Fieuws S, Penninckx F. Effect of centralization of pancreaticoduodenectomy on nationwide hospital mortality and length of stay. Br J Surg. 2007; 94:1377–1381.

16. Balcom JH 4th, Rattner DW, Warshaw AL, Chang Y, Fernandez-del Castillo C. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001; 136:391–398.
17. Traverso LW, Shinchi H, Low DE. Useful benchmarks to evaluate outcomes after esophagectomy and pancreaticoduodenectomy. Am J Surg. 2004; 187:604–608.

18. Schmidt CM, Powell ES, Yiannoutsos CT, Howard TJ, Wiebke EA, Wiesenauer CA, et al. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg. 2004; 139:718–725.
19. Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006; 10:1199–1210.

20. Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006; 244:10–15.

21. Chew DK, Attiyeh FF. Experience with the Whipple procedure (pancreaticoduodenectomy) in a university-affiliated community hospital. Am J Surg. 1997; 174:312–315.

22. Kim H, Chung JK, Ahn YJ, Lee HW, Jung IM. The 13-year experience of performing pancreaticoduodenectomy in a mid-volume municipal hospital. Ann Surg Treat Res. 2017; 92:73–81.

23. Cooperman AM. Pancreatic cancer: the bigger picture. Surg Clin North Am. 2001; 81:557–574.
24. Afsari A, Zhandoug Z, Young S, Ferguson L, Silapaswan S, Mittal V. Outcome analysis of pancreaticoduodenectomy at a community hospital. Am Surg. 2002; 68:281–284.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download