1. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001; 285:785–795. PMID:
11176917.
2. Park EJ, Joo IW, Jang MJ, Kim YT, Oh K, Oh HJ. Prevalence of osteoporosis in the Korean population based on Korea National Health and Nutrition Examination Survey (KNHANES), 2008–2011. Yonsei Med J. 2014; 55:1049–1057. PMID:
24954336.

3. Demir B, Haberal A, Geyik P, Baskan B, Ozturkoglu E, Karacay O, et al. Identification of the risk factors for osteoporosis among postmenopausal women. Maturitas. 2008; 60:253–256. PMID:
18778903.

4. Salari P, Abdollahi M. The influence of pregnancy and lactation on maternal bone health: a systematic review. J Family Reprod Health. 2014; 8:135–148. PMID:
25530765.
5. Oliveri B, Parisi MS, Zeni S, Mautalen C. Mineral and bone mass changes during pregnancy and lactation. Nutrition. 2004; 20:235–240. PMID:
14962693.

6. Tsvetov G, Levy S, Benbassat C, Shraga-Slutzky I, Hirsch D. Influence of number of deliveries and total breast-feeding time on bone mineral density in premenopausal and young postmenopausal women. Maturitas. 2014; 77:249–254. PMID:
24332872.

7. Sharma N, Natung T, Barooah R, Ahanthem SS. Effect of multiparity and prolonged lactation on bone mineral density. J Menopausal Med. 2016; 22:161–166. PMID:
28119896.

8. To WW, Wong MW. Changes in bone mineral density of the os calcis as measured by quantitative ultrasound during pregnancy and 24 months after delivery. Aust N Z J Obstet Gynaecol. 2011; 51:166–171. PMID:
21466520.
9. Streeten EA, Ryan KA, McBride DJ, Pollin TI, Shuldiner AR, Mitchell BD. The relationship between parity and bone mineral density in women characterized by a homogeneous lifestyle and high parity. J Clin Endocrinol Metab. 2005; 90:4536–4541. PMID:
15899951.

10. Hiz O, Ediz L, Tekeoglu I. Effect of number of pregnancies on bone mineral density. J Int Med Res. 2010; 38:1816–1823. PMID:
21309498.

11. Lenora J, Lekamwasam S, Karlsson MK. Effects of multiparity and prolonged breast-feeding on maternal bone mineral density: a community-based cross-sectional study. BMC Womens Health. 2009; 9:19. PMID:
19570205.

12. Kojima N, Douchi T, Kosha S, Nagata Y. Cross-sectional study of the effects of parturition and lactation on bone mineral density later in life. Maturitas. 2002; 41:203–209. PMID:
11886766.

13. Hopkinson JM, Butte NF, Ellis K, Smith EO. Lactation delays postpartum bone mineral accretion and temporarily alters its regional distribution in women. J Nutr. 2000; 130:777–783. PMID:
10736329.

14. Okyay DO, Okyay E, Dogan E, Kurtulmus S, Acet F, Taner CE. Prolonged breast-feeding is an independent risk factor for postmenopausal osteoporosis. Maturitas. 2013; 74:270–275. PMID:
23352271.

15. Yilmaz H, Erkin G, Polat HAD, Küçüksen S, Sallı A, Uğurlu H. Effects of reproductive factors on bone mineral densitometry. Turk J Osteoporos. 2012; 18:8–12.
16. Hadji P, Ziller V, Kalder M, Gottschalk M, Hellmeyer L, Hars O, et al. Influence of pregnancy and breast-feeding on quantitative ultrasonometry of bone in postmenopausal women. Climacteric. 2012; 5:277–285.

17. Yazici S, Korkmaz U, Erkan M, Korkmaz N, Erdem Baki A, Alçelik A, et al. The effect of breast-feeding duration on bone mineral density in postmenopausal Turkish women: a population-based study. Arch Med Sci. 2011; 7:486–492. PMID:
22295033.

18. Wang Q, Huang Q, Zeng Y, Liang JJ, Liu SY, Gu X, et al. Parity and osteoporotic fracture risk in postmenopausal women: a dose-response meta-analysis of prospective studies. Osteoporos Int. 2016; 27:319–330. PMID:
26439242.

19. Song SY, Kim Y, Park H, Kim YJ, Kang W, Kim EY. Effect of parity on bone mineral density: a systematic review and meta-analysis. Bone. 2017; 101:70–76. PMID:
28450215.

20. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa (ON): Ottawa Hospital Research Institute;cited 2019 Jan 5. Available from:
http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
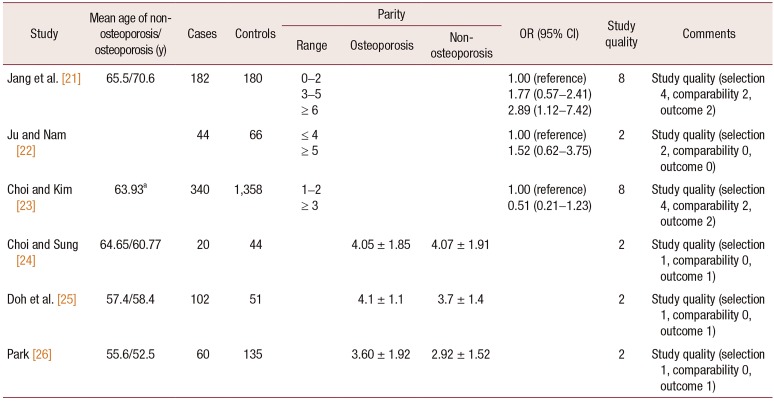
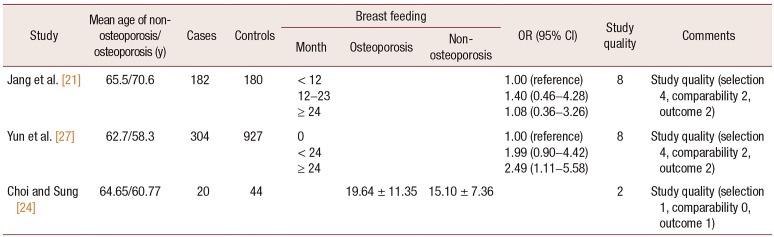
21. Jang SN, Choi YH, Choi MG, Kang SH, Jeong JY, Choi YJ, et al. Prevalence and associated factors of osteoporosis among postmenopausal women in Chuncheon: Hallym Aging Study (HAS). J Prev Med Public Health. 2006; 39:389–396. PMID:
17076179.
22. Ju MS, Nam SL. A study on risk factors of osteoporosis. J Rheumatol Health. 1999; 6:37–50.
23. Choi KJ, Kim KH. Factors influencing bone mineral density by postmenopausal ages. Korean J Health Serv Manag. 2017; 11:145–155.

24. Choi YH, Sung CJ. Effects of physiological factors and lifestyles on bone mineral density in postmenopausal women. Korean J Nutr. 2007; 40:517–525.
25. Doh JH, Kang PS, Joo R, Kim SB, Kim SK. Determinants of bone mineral density in adult women. J Korean Soc Matern Child Health. 2000; 4:189–198.
26. Park MH. Risk factors on osteoporosis in the menopausal women [Master's thesis]. Seoul: Ewha Womans University;1995.
27. Yun BH, Chon SJ, Choi YS, Cho S, Lee BS, Seo SK. The effect of prolonged breast-feeding on the development of postmenopausal osteoporosis in population with insufficient calcium intake and vitamin D level. Osteoporos Int. 2016; 27:2745–2753. PMID:
27048389.

28. Duan X, Wang J, Jiang X. A meta-analysis of breastfeeding and osteoporotic fracture risk in the females. Osteoporos Int. 2017; 28:495–503. PMID:
27577724.

29. Bayray A, Enquselassie F. The effect of parity on bone mineral density in postmenopausal women: a systematic review. J Osteopor Phys Act. 2013; 1:1–6.
30. Chowdhury R, Sinha B, Sankar MJ, Taneja S, Bhandari N, Rollins N, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015; 104:96–113. PMID:
26172878.

31. Naz MSG, Ghasemi V, Kiani Z, Fakari FR, Ozgoli G. The effect of breastfeeding duration on bone mineral density (BMD): a systematic review and meta-analysis. Int J Pediatr. 2019; 7:8831–8843.
32. Kovacs CS. Calcium and bone metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia. 2005; 10:105–118. PMID:
16025218.

33. World Health Organization. Infant and young child feeding: model chapter for textbooks for medical students and allied health professionals. Geneva: World Health Organization;2009.
34. Kovacs CS. Calcium and bone metabolism in pregnancy and lactation. J Clin Endocrinol Metab. 2001; 86:2344–2348. PMID:
11397820.

35. Hwang IR, Choi YK, Lee WK, Kim JG, Lee IK, Kim SW, et al. Association between prolonged breastfeeding and bone mineral density and osteoporosis in postmenopausal women: KNHANES 2010–2011. Osteoporos Int. 2016; 27:257–265. PMID:
26373982.

36. More C, Bettembuk P, Bhattoa HP, Balogh A. The effects of pregnancy and lactation on bone mineral density. Osteoporos Int. 2001; 12:732–737. PMID:
11605738.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download