INTRODUCTION
METHODS
Cell culture
Intracellular calcium measurement
Western blot
Statistical analysis
RESULTS
Dose-response effect of DHA on ATP-induced intracellular calcium concentration
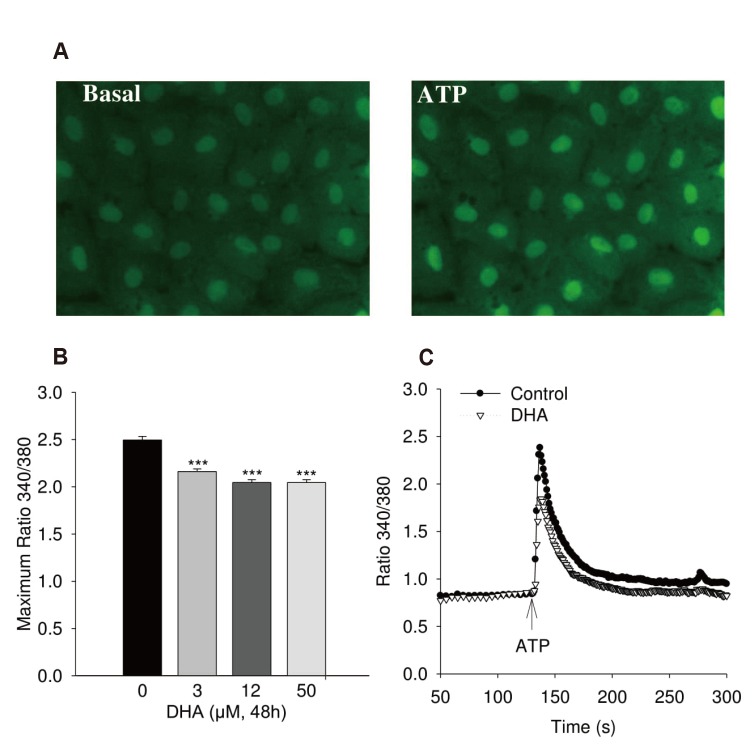 | Fig. 1Calcium increase of human umbilical vein endothelial cell (HUVEC) induced by adenosine triphosphate (ATP).(A) Typical fluorescence image. Typical fluorescence of Fura-2AM loading cell image with 40× magnification. (B) Maximum of ATP (100 µM) induced calcium transient in HUVEC treated with 0, 3, 12, 50 µmol/l docosahexaenoic acid (DHA) (n = 4). (C) Kinetic of calcium transient in cells stimulated with ATP (100 µM) without and with treatment with 12 µM DHA for 48 h (n = 8). Cells were loaded with 5 µM Fura-2-AM. ANOVA; ***p < 0.0001 between DHA treatment and control.
|
Effect of DHA on ATP-induced eNOS phosphorylation
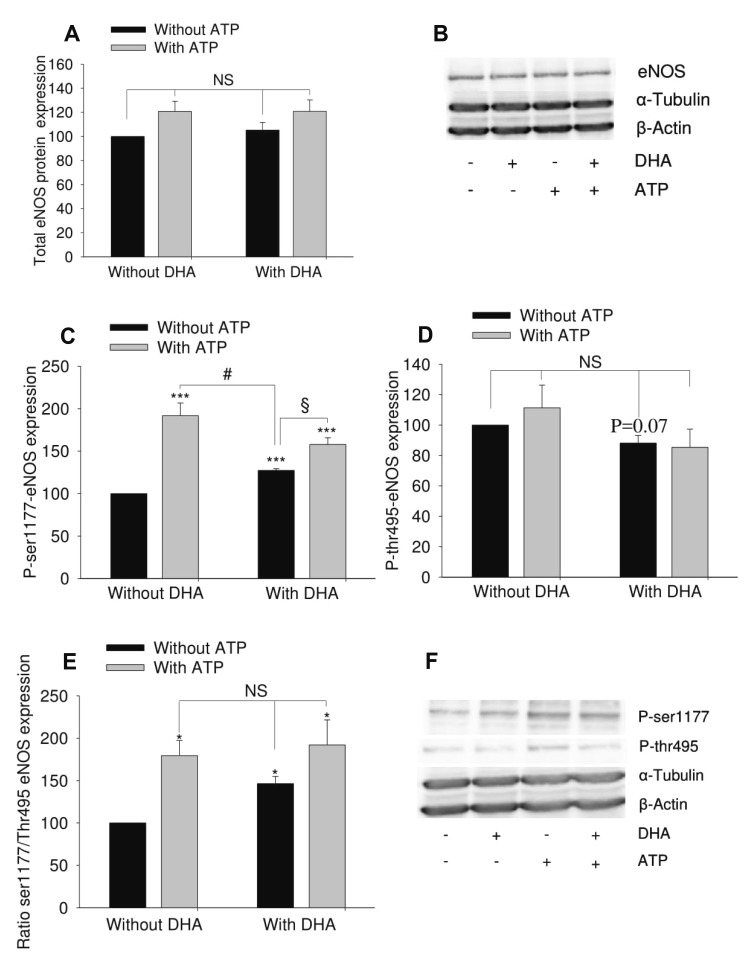 | Fig. 2Effect of docosahexaenoic acid (DHA) on adenosine triphosphate (ATP)-induced endothelial nitric oxide synthase (eNOS) phosphorylation in human umbilical vein endothelial cell (HUVEC).HUVEC were treated with 12 µM DHA for 48 h. 100 µM ATP was used to stimulate eNOS for 1 min. (A) Total eNOS expression (n = 7). (B) Representative blot. (C) Phosphorylated eNOS at ser1177 residue (n = 5). (D) Phosphorylated eNOS at thr495 residue (n = 5). (E) Ratio of phosphorylated eNOS at ser1177 and phosphorylated eNOS at thr495 (n = 5). (F) Representative Western blot. The results show mean ± standard error of densitometric quantification of blots. ANOVA was used to test for differences. *p < 0.05, ***p < 0.001 vs. untreated group (no DHA, no ATP); #p < 0.05, §p < 0.05. NS, no significant difference.
|
Effect of DHA on ATP-induced calcium increase depends on extracellular calcium
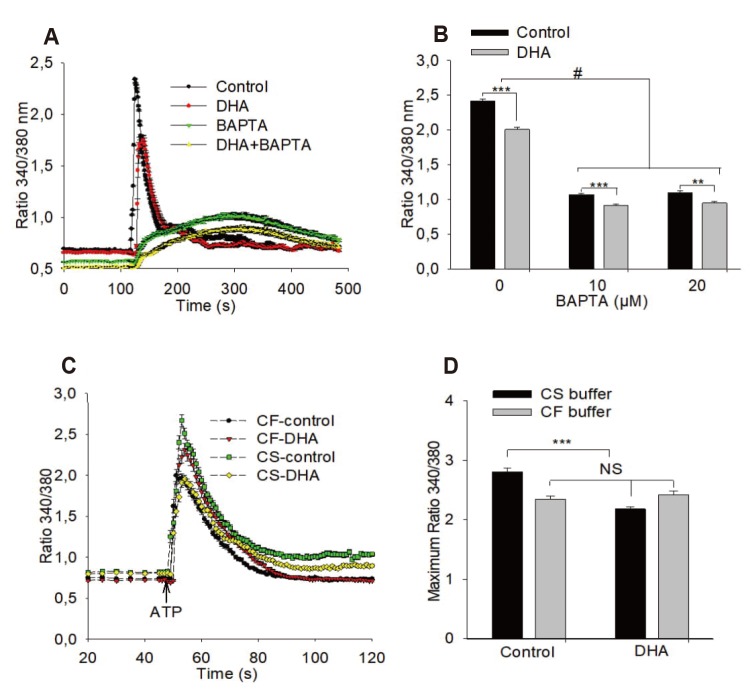 | Fig. 3Effects of BAPTA and withdrawal of extracellular calcium on adenosine triphosphate (ATP) induced calcium transient.(A, B) Presence of BAPTA (10 µM, 20 µM) does not eliminate the effect of docosahexaenoic acid (DHA) on ATP-induced calcium transient (n = 3). (C, D) Elimination of extracellular calcium reduces the ATP-induced calcium transient and abolishes the effect of DHA on the calcium transient (n = 4). DHA 12 µM, ATP 100 µM; ANOVA/Tukey's post-hoc test was used: **p < 0.001; ***p < 0.0001, #p < 0.0001 between groups as indicated by brackets,. NS, no significant difference; CS, calcium-saturated; CF, calcium-free. For further information see methods.
|
DHA inhibits ATP-induced calcium influx via store-operated calcium channels that activate eNOS
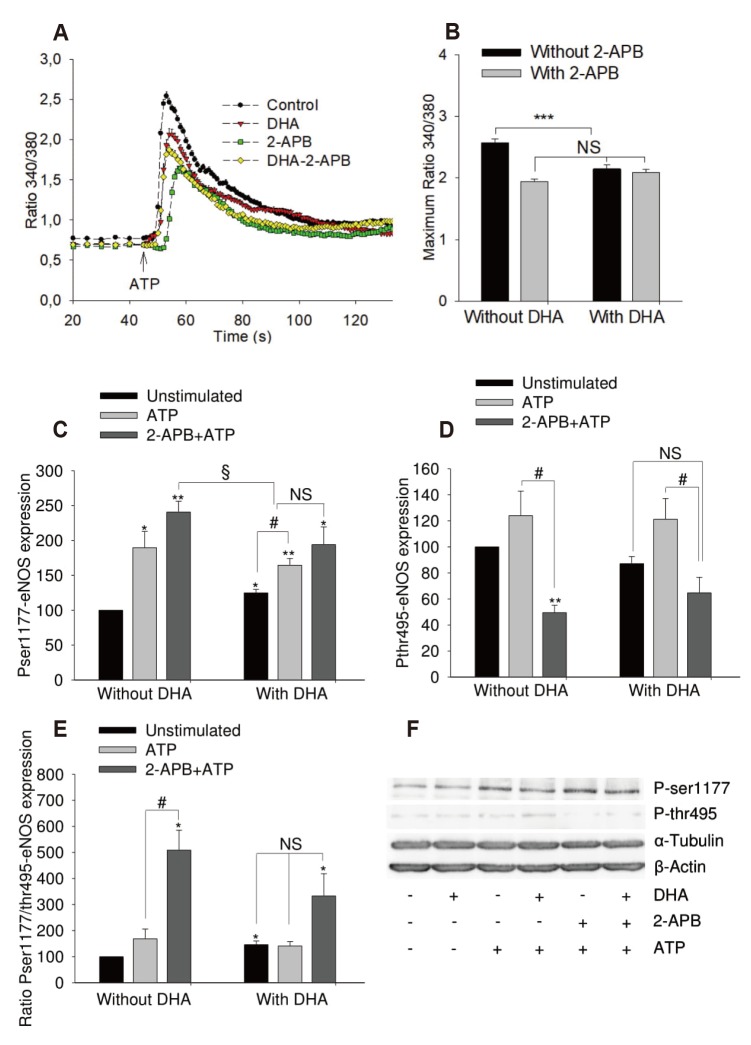 | Fig. 4Effects of 2-aminoethoxydiphenyl borate (2-APB) on adenosine triphosphate (ATP) induced calcium transient and endothelial nitric oxide synthase (eNOS) phosphorylation of human umbilical vein endothelial cell.Cells were left untreated or treated with 12 µM of docosahexaenoic acid (DHA) for 48 h. (A, B) Effect of 2-APB (75 µM, 30 min at 37℃) on ATP-induced (100 µM) calcium increase. ANOVA/Tukey's post-hoc test, n = 4, ***p < 0.0001 vs. untreated group. (C–F) Effects on eNOS phosphorylation. Results show mean ± standard error of densitometric quantification of blots from 4 independent experiments. ANOVA was used to test for differences between groups. *p < 0.05, **p < 0.01 vs. untreated group; #p < 0.05, §p < 0.05. NS, no significant difference.
|
Effect of calcium store depletion by thapsigargin on calcium signal and eNOS phosphorylation
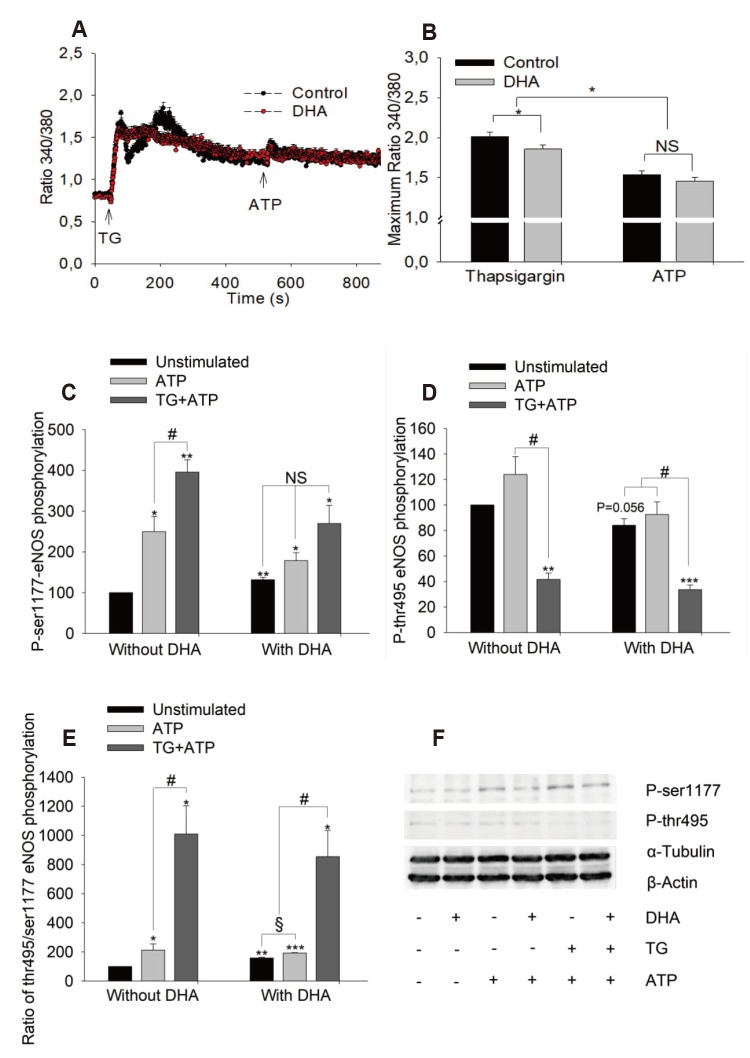 | Fig. 5Effects of calcium store-depletion by thapsigargin on adenosine triphosphate (ATP)-induced maximum calcium increase and endothelial nitric oxide synthase (eNOS) phosphorylation.(A, B) Maximum of calcium increase in presence of thapsigargin (2 µM) on the left and following ATP (100 µM) in presence of thapsigargin on the right. The calcium signal was recorded following thapsigargin (TG) for 450 sec before ATP was applied. Cells were either treated with docosahexaenoic acid (DHA) 12 µM for 48 h or left untreated (control), n = 3, 37℃. (C–F) Effects of TG on eNOS phosphorylation. Cells were stimulated with TG (1 µM, 10 min) before stimulation with ATP (100 µM, 1 min). Samples were harvested and stored at −80℃ for western blot. Results show mean ± standard error, n = 4. ANOVA/Tukey's post-hoc test was used to test for differences between groups. *p < 0.05, **p < 0.01, ***p < 0.001 vs. untreated group; #p < 0.05, §p < 0.05. NS, no significant difference.
|
Evidence for a P2R-mediated effect after ATP stimulation
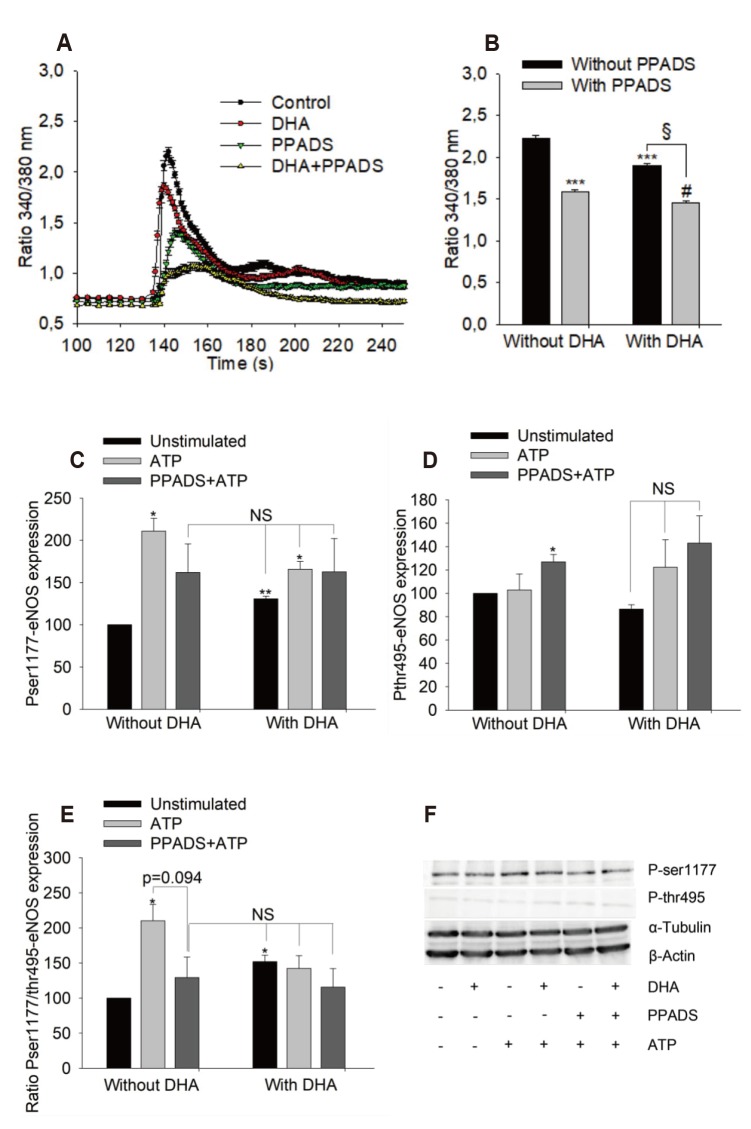 | Fig. 6Effects of PPADS on adenosine triphosphate (ATP)-induced maximum calcium signal and endothelial nitric oxide synthase (eNOS) phosphorylation.(A, B) Maximum calcium signal in human umbilical vein endothelial cell treated with 12 µM docosahexaenoic acid (DHA) for 48 h or left untreated (control), n = 3, PPADS 100 µM for 30 min at 37℃. (C–F) Effects of PPADS on eNOS phosphorylation. Results show mean ± standard error, n = 3. Data was tested using ANOVA/Tukey's post-hoc test to compare different groups. *p < 0.05, **p < 0.01, ***p < 0.0001 vs. untreated group; §p < 0.0001 between DHA treated groups with and without PPADS; #p < 0.01 between PPADS treated groups with and without DHA. NS, no significant difference.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download