1. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010; 362:2155–2165. PMID:
20558366.

2. French JK, White H. Clinical implications of the new definition of myocardial infarction. Heart. 2004; 90:99–106. PMID:
14676259.

3. Hassan AK, Bergheanu SC, Hasan-Ali H, Liem SS, van der Laarse A, Wolterbeek R, et al. Usefulness of peak troponin-T to predict infarct size and long-term outcome in patients with first acute myocardial infarction after primary percutaneous coronary intervention. Am J Cardiol. 2009; 103:779–784. PMID:
19268731.

4. Chia S, Senatore F, Raffel OC, Lee H, Wackers FJ, Jang IK. Utility of cardiac biomarkers in predicting infarct size, left ventricular function, and clinical outcome after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2008; 1:415–423. PMID:
19463339.

5. Ohlmann P, Jaquemin L, Morel O, El Behlgiti R, Faure A, Michotey MO, et al. Prognostic value of C-reactive protein and cardiac troponin I in primary percutaneous interventions for ST-elevation myocardial infarction. Am Heart J. 2006; 152:1161–1167. PMID:
17161070.

6. Min JY, Ko SM, Song IY, Yi JG, Hwang HK, Shin JK. Comparison of the diagnostic accuracies of 1.5T and 3T stress myocardial perfusion cardiovascular magnetic resonance for detecting significant coronary artery disease. Korean J Radiol. 2018; 19:1007–1102. PMID:
30386133.

7. Yan AT, Gibson CM, Larose E, Anavekar NS, Tsang S, Solomon SD, et al. Characterization of microvascular dysfunction after acute myocardial infarction by cardiovascular magnetic resonance first-pass perfusion and late gadolinium enhancement imaging. J Cardiovasc Magn Reson. 2006; 8:831–837. PMID:
17060106.

8. Mather AN, Lockie T, Nagel E, Marber M, Perera D, Redwood S, et al. Appearance of microvascular obstruction on high resolution first-pass perfusion, early and late gadolinium enhancement CMR in patients with acute myocardial infarction. J Cardiovasc Magn Reson. 2009; 11:33. PMID:
19698105.

9. Nguyen TL, French JK, Hogan J, Hee L, Moses D, Mussap CJ, et al. Prognostic value of high sensitivity troponin T after ST-segment elevation myocardial infarction in the era of cardiac magnetic resonance imaging. Eur Heart J Qual Care Clin Outcomes. 2016; 2:164–171. PMID:
29474609.

10. Chung HW, Ko SM, Hwang HK, So Y, Yi JG, Lee EJ. Diagnostic performance of coronary CT angiography, stress dual-energy CT perfusion, and stress perfusion single-photon emission computed tomography for coronary artery disease: comparison with combined invasive coronary angiography and stress perfusion cardiac MRI. Korean J Radiol. 2017; 18:476–486. PMID:
28458600.

11. Rossi A, Merkus D, Klotz E, Mollet N, de Feyter PJ, Krestin GP. Stress myocardial perfusion: imaging with multidetector CT. Radiology. 2014; 270:25–46. PMID:
24354374.

12. Danad I, Szymonifka J, Schulman-Marcus J, Min JK. Static and dynamic assessment of myocardial perfusion by computed tomography. Eur Heart J Cardiovasc Imaging. 2016; 17:836–844. PMID:
27013250.

13. Coenen A, Rossi A, Lubbers MM, Kurata A, Kono AK, Chelu RG, et al. Integrating CT myocardial perfusion and CT-FFR in the work-up of coronary artery disease. JACC Cardiovasc Imaging. 2017; 10:760–770. PMID:
28109933.
14. Rossi A, Wragg A, Klotz E, Pirro F, Moon JC, Nieman K, et al. Dynamic computed tomography myocardial perfusion imaging: comparison of clinical analysis methods for the detection of vessel-specific ischemia. Circ Cardiovasc Imaging. 2017; 10:pii: e005505.
15. Pelgrim GJ, Duguay TM, Stijnen JM, Varga-Szemes A, Van Tuijl S, Schoepf UJ, et al. Analysis of myocardial perfusion parameters in an ex-vivo porcine heart model using third generation dual-source CT. J Cardiovasc Comput Tomogr. 2017; 11:141–147. PMID:
28202246.

16. Bamberg F, Klotz E, Flohr T, Becker A, Becker CR, Schmidt B, et al. Dynamic myocardial stress perfusion imaging using fast dual-source CT with alternating table positions: initial experience. Eur Radiol. 2010; 20:1168–1173. PMID:
20333388.

17. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the cardiac imaging committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002; 105:539–542. PMID:
11815441.

18. Kono AK, Coenen A, Lubbers M, Kurata A, Rossi A, Dharampal A, et al. Relative myocardial blood flow by dynamic computed tomographic perfusion imaging predicts hemodynamic significance of coronary stenosis better than absolute blood flow. Invest Radiol. 2014; 49:801–807. PMID:
25014013.

19. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440–1463. PMID:
16376782.

20. Nakamura S, Kitagawa K, Goto Y, Omori T, Kurita T, Yamada A, et al. Incremental prognostic value of myocardial blood flow quantified with stress dynamic computed tomography perfusion imaging. JACC Cardiovasc Imaging. 2018; 7. 13. DOI:
10.1016/j.jcmg.2018.05.021. [Epub ahead of print].
21. Meinel FG, Wichmann JL, Schoepf UJ, Pugliese F, Ebersberger U, Lo GG, et al. Global quantification of left ventricular myocardial perfusion at dynamic CT imaging: prognostic value. J Cardiovasc Comput Tomogr. 2017; 11:16–24. PMID:
28111212.

22. Niccoli G, Scalone G, Lerman A, Crea F. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J. 2016; 37:1024–1033. PMID:
26364289.

23. So A, Hsieh J, Li JY, Hadway J, Kong HF, Lee TY. Quantitative myocardial perfusion measurement using CT perfusion: a validation study in a porcine model of reperfused acute myocardial infarction. Int J Cardiovasc Imaging. 2012; 28:1237–1248. PMID:
21800119.

24. van Kranenburg M, Magro M, Thiele H, de Waha S, Eitel I, Cochet A, et al. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging. 2014; 7:930–939. PMID:
25212799.
25. Hamirani YS, Wong A, Kramer CM, Salerno M. Effect of microvascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial infarction: a systematic review and meta-analysis. JACC Cardiovasc Imaging. 2014; 7:940–952. PMID:
25212800.
26. Pernet K, Ecarnot F, Chopard R, Seronde MF, Plastaras P, Schiele F, et al. Microvascular obstruction assessed by 3-tesla magnetic resonance imaging in acute myocardial infarction is correlated with plasma troponin I levels. BMC Cardiovasc Disord. 2014; 14:57. PMID:
24886208.

27. Bamberg F, Marcus RP, Becker A, Hildebrandt K, Bauner K, Schwarz F, et al. Dynamic myocardial CT perfusion imaging for evaluation of myocardial ischemia as determined by MR imaging. JACC Cardiovasc Imaging. 2014; 7:267–277. PMID:
24529887.

28. Mahnken AH, Klotz E, Pietsch H, Schmidt B, Allmendinger T, Haberland U, et al. Quantitative whole heart stress perfusion CT imaging as noninvasive assessment of hemodynamics in coronary artery stenosis: preliminary animal experience. Invest Radiol. 2010; 45:298–305. PMID:
20421799.
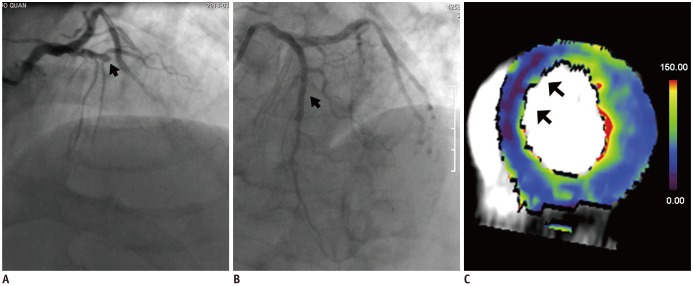
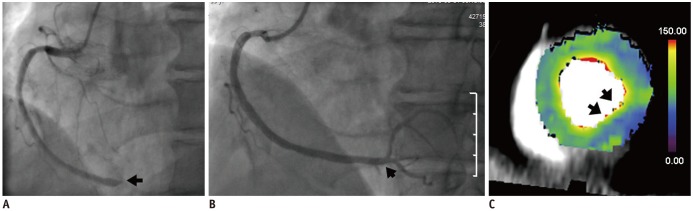
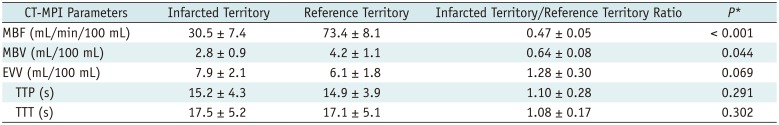
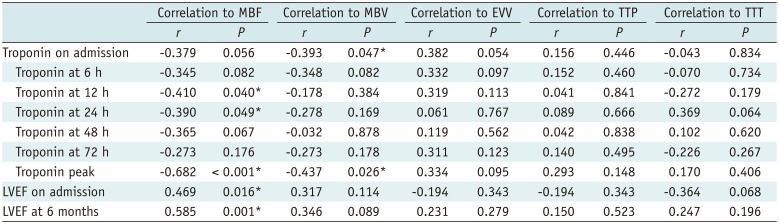




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download