INTRODUCTION
Iterative reconstruction (IR) technique is an important radiation dose reduction strategy and could achieve radiation dose reduction without significant compromise of image quality, compared with filtered back projection (FBP) of routine radiation dose (
1234567). The iterative noise reduction process can be performed in different domains (in image domain alone, sinogram domain alone, or both domains) depending on IR techniques (
78). Computed tomography (CT) vendors have adopted different algorithms according to their application choices and development stages, which led to a variety of vendor-specific IR techniques in practice. Hybrid IR, which uses the relevant information in both the sinogram and image domains, is the most widely used technique in vendors' IR packages today because of its superior performance and computational efficiency (
7).
However, using individual vendor-specific IR techniques in a setting of multiple CT units from different vendors may be more expensive than using vendor-neutral IR packages (
3). Another critical problem is unavailability of vendor-specific IR techniques compatible with outdated CT machines in current operation. In this situation, vendor-neutral IR techniques can be a good solution to decrease radiation exposure without compromising daily workflow, if vendor-neutral IR techniques are comparable to vendor-specific techniques.
Its applicability would be greater in young patients, because radiation dose reduction is a major issue in these patients due to their vulnerability to radiation (
910). However, to the best of our knowledge, no study has compared the image quality between vendor-neutral and vendor-specific IR techniques in young patients.
ClariCT (ClariPI, Seoul, Korea) is a commercially available vendor-neutral IR technique, which features digital imaging and communications in medicine (DICOM)-based hybrid IR. ClariCT shares the same procedures of the hybrid IR as used in vendor-specific hybrid IR techniques with the advantage of superior noise reduction performance compared with image-based techniques: reduction of sinogram specific noises that are more easily captured in sinogram-domain such as salient local variations of attenuation coefficients on highly attenuated ray paths, followed by additional enhancement steps in image-domain such as edge-adaptive blending of image sub-components. Unlike the vendor-specific hybrid IR, ClariCT includes an additional step of sinogram synthesis from DICOM CT image which is not required in vendor-specific IR techniques. A sophisticated sinogram synthesis procedure including the use of appropriate scanner geometry and advanced forward and backward projection techniques is regarded the key element enabling its denoising performance to potentially match with the vendor-specific hybrid IR techniques (
1112). Information about scanner geometry was obtained from both DICOM header and open sources such as “
http://www.impactsca.org/.” Thus, ClariCT may be unique in potentially providing enhanced denoising performance and convenient vendor-neutral technique.
This study aimed to evaluate the image quality of the vendor-neutral IR technique and compare it with that of FBP and vendor-specific hybrid IR technique in abdominopelvic CT for young patients through phantom and clinical studies.
MATERIALS AND METHODS
Our Institutional Review Board (IRB) approved this retrospective study and waived the requirement for informed consent.
Phantom
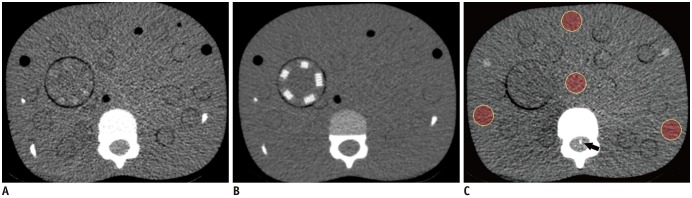
In the phantom study, a 5-year-old age-equivalent anthropomorphic pediatric phantom (ATOM model 705; CIRS, Norfolk, VA, USA) and a CT imaging quality assurance kit (Model 700-QA; CIRS) made of tissue equivalent epoxy resins were used. The CT image quality assurance kit included two soft-tissue inserts of cylindrical and line pair targets; they were positioned in the upper abdomen of the pediatric phantom (
Fig. 1). The height and axial dimension of the pediatric phantom was 110 cm and 17.0 × 14.0 cm at the level of the upper abdomen, respectively. The calculated effective diameter was 15.4 cm (
131415). Details of phantom and soft-tissue inserts are follows: 1) The soft-tissue insert with cylindrical targets is composed of 18 low-contrast targets (with 6 variable sizes, ranging from 1.2 mm to 7 mm) with 20 Hounsfield unit (HU) contrast higher than the background (
16). 2) The soft-tissue insert with line pair targets is made up of five targets (6, 8, 10, 11, and 12 line pairs per centimeter) of which the attenuation (HU) is 300 HU higher than the background (
16).
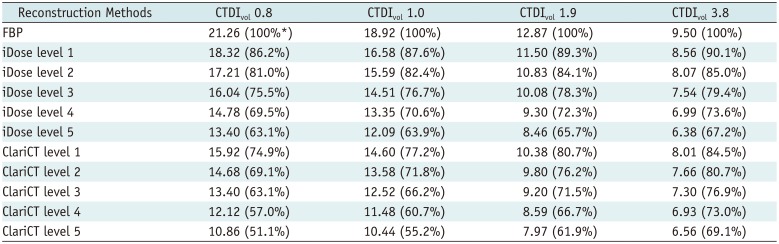
The pediatric phantom was scanned through the 256-slice CT scanner (iCT 256; Philips Healthcare, Andover, MA, USA) with an effective tube current-time product of 19, 24, 47, and 93 mAs at a kilovoltage of 100 kVp. The volume CT dose index (CTDIvol) was 0.8, 1.0, 1.9, and 3.8 mGy, respectively. The other scanning parameters were as follows: detector collimation: 0.625 × 40 mm, gantry rotation time: 0.27 seconds, and pitch: 0.617. Automatic tube current modulation was not applied for the phantom study.
The CT images were reconstructed for each scan using traditional FBP and vendor-specific IR (iDose; Philips Healthcare) with five different IR strengths. FBP and iDose images were reconstructed using a standard routine body reconstruction filter (B filter; Philips Healthcare). Images were also reconstructed using vendor-neutral reconstruction technique (ClariCT) with five different levels of reconstruction strength from the FBP images. The other image reconstruction parameters were the same throughout: display field of view, 220 × 220 mm; image matrix, 512 × 512; slice thickness, 3 mm; and slice overlap, 1 mm. For the ClariCT, mean denoising time per slice was 0.3 seconds, and overall transfer and processing time of an exam was 2–3 minutes.
For quantitative analyses of image noise, a single radiologist (with 3 years' experience with CT) selected one image of the same level at the upper abdomen from each of the reconstructions at different radiation dose levels. Four regions of interest (ROIs) were drawn on each image (
Fig. 1) at the picture archiving and communication system (PACS) workstation (Infinitt; Infinitt Healthcare, Seoul, Korea) with displays calibrated to the DICOM grayscale standard display function. One ROI was located at the center of the phantom, and three were at the peripheral portion of the phantom, as previously reported (
15). The ROI area was constant at 200 mm
2. The image noise for each reconstruction series was the mean of four standard deviation (SD) values measured from four ROIs.
In the quantitative analyses of spatial resolution, images obtained at the CTDI
vol of 1.9 mGy were used. Modulation transfer function (MTF) value was obtained using the wire located in the spinal canal (
Fig. 1) with in-house software based on the method reported by Nickoloff (
17). Image pixels within an ROI on the wire were subtracted from the ROI-mean, followed by Fourier transform and radial sampling. ROI size of 16 pixels was used to reduce noise effect (
17). The spatial frequency at 10% MTF (MTF
10) was used to assess the spatial resolution limit.
Noise power spectrum (NPS) was also evaluated to determine the noise content of images using an in-house software based on the method described by Baek and Pelc (
18). Image pixels on an ROI of 32-pixel size placed on a uniform central area were subtracted from the ROI-mean, followed by Fourier transform, square function of the magnitude component, and normalization with the ROI area (
18).
In the qualitative analyses of imaging parameters, two radiologists (with 3 and 13 years' experience with CT, respectively) reviewed images obtained at the CTDI
vol level of 1.9 mGy, in consensus, on the same PACS workstation. The cylindrical target was used for evaluation of low-contrast resolution. In the optimized liver setting (window width, 150 HU; window level, 50 HU) (
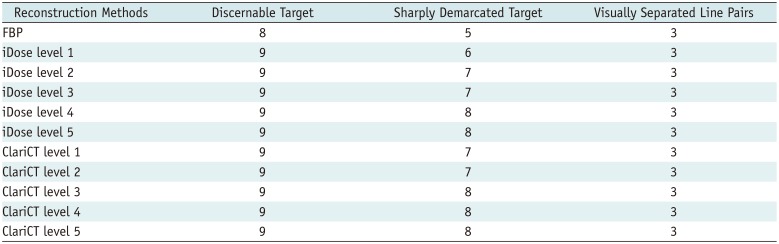
19), the number of discernible targets and that of sharply defined cylindrical targets were evaluated for low-contrast resolution (
20). Using the line pair target, assessment of subjective spatial resolution was performed by counting the number of line pairs, wherein adjacent lines were considered separate lines, with window width of 500 HU and level of 100 HU.
Clinical Data
From August 2015 to April 2016, patients who underwent abdominopelvic CT through multi-detector CT (iCT 256) with available both FBP and iDose reconstruction images were included in the study. A total of 43 young patients (26 males and 17 females; median age, 14 years; age range, 1–19 years) were included. The mean effective diameter at the superior mesenteric artery os level was 21.0 ± 4.6 cm, ranging from 13.1 to 33.2 cm (
13). The clinical indications for abdominal CT and clinically relevant positive findings are described in
Supplementary Table 1.
The median CTDIvol at 32 cm was 1.7 mGy (range, 0.6–10.7 mGy), and kVp was ranging from 80 to 140 kVp (10 studies using 80 kVp, 31 using 100 kVp, 1 using 120 kVp, and 1 using 140 kVp). Automated tube current modulation was adapted. Slice thickness was 3 mm, detector collimation was 0.625 × 40 mm, pitch was 0.617, gantry rotation time was 0.27 seconds, and Dose Right Index, which is a discrete parameter designed to make a consistent image quality for every patient, was 14.
In our institution, all iDose images were reconstructed at strength level 4 (iDose4), because CT images with iDose4 were thought to be optimal level considering both image qualities and noise, as Karmazyn et al. (
21) described.
In quantitative and qualitative comparisons between vendor-neutral and vendor-specific IR techniques, ClariCT images were reconstructed from the FBP images, at the denoising level that was similar to that of clinically used vendor-specific IR images (i.e., iDose4), based on the phantom study results.
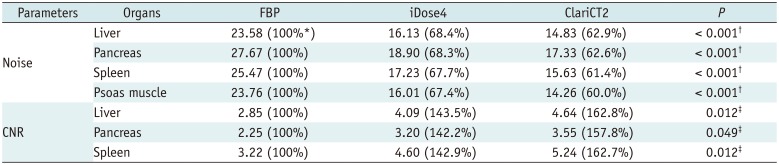
For quantitative analysis, noise and contrast-to-noise ratio (CNR) were calculated by drawing ROIs in the liver (four ROIs; mean area, 254.1 mm
2; range, 187.3–311.34 mm
2), pancreas (three ROIs; mean area, 157.3 mm
2; range, 68.4–221.6 mm
2), spleen (two ROIs; mean area, 246.3 mm
2; range, 129.9–343.2 mm
2), and two psoas muscles (single ROI; mean area, 234.1 mm
2; range, 154.3–338.8 mm
2) on FBP, iDose, and ClariCT images by a single radiologist (
Supplementary Fig. 1). Large vessels, pancreatic ducts, macroscopic fat infiltration, and focal lesions were carefully avoided. Mean attenuation (HU) and SD of each organ were calculated.
The CNRs of the liver, pancreas, and spleen, relative to the muscle were calculated using the following equations: CNR = (mHU
o − mHU
m) / SD
m, where mHU
o is the mean attenuation in the organ of interest, mHU
m is the mean attenuation in the paraspinal muscles, and SD
m is the image noise of the paraspinal muscle (
22).
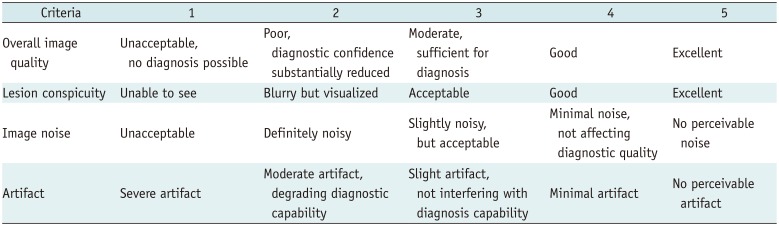
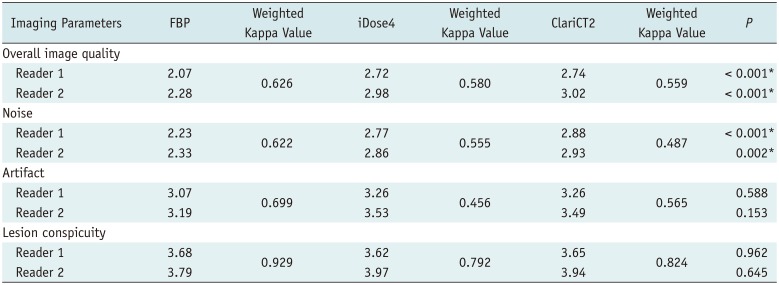
For qualitative comparisons of imaging parameters, overall image quality, lesion conspicuity, image noise, and image artifact such as streak and beam hardening artifacts were evaluated by two radiologists (
Table 1). Overall image quality was scored using a 5-point Likert scale (
23). Lesion conspicuity was evaluated in 34 patients with lesions over 5 mm using a 5-point scale (
24). The details of lesions in these 34 patients are described in
Supplementary Table 2. Image noise and artifact were also rated (
25).
Statistical Analysis
To evaluate the normal distribution of parameters, D'Agostino-Pearson test for normal distribution was performed. For comparison of quantitative noise, CNR, and qualitative image quality scores, one-way repeated measures ANOVA was performed, followed by a post-hoc test using pairwise comparison with Bonferroni correction provided by statistical software.
Interobserver reliability for subjective scoring was assessed by using linear weighted kappa coefficient graded as follows: none to slight, < 0.20; fair, 0.21–0.40; moderate, 0.41–0.60; substantial, 0.61–0.80; and excellent, > 0.80 (
26).
All statistical analyses were performed using MedCalc (Version 15.2; MedCalc Software, Ostend, Belgium). A p value of less than 0.05 was considered statistically significant.
DISCUSSION
ClariCT is a vendor-neutral IR technique which features DICOM-based sinogram synthesis and hybrid IR and has advantage of both sinogram-based and image-based denoising. The hybrid algorithm in ClariCT is performed in a unique arrangement. The first step is forward projection of an FBP-generated CT image to create a synthesized sinogram, which is the same part shared in most hybrid reconstruction techniques. Here, the geometry of the CT system of interest is derived indirectly from the DICOM header and related literature. In the second step, the algorithm analyzes the synthesized sinogram and identifies the noisiest part of sinogram (photon-starved area). This is followed by an extraction of noise sinogram through an iterative process with irregularity and an unusual statistical pattern. Then FBP is applied to the noise sinogram and reconstructs a noise CT image, which mostly contains streak and irregular directional noises. Subsequently, an additional processing loop is applied in image space to further reduce the noise left after subtraction of the reconstructed noise CT. Finally, the denoised image is adaptively blended with the original FBP image by using the local noise statistic, which attempts to avoid the plastic appearance of the processed image due to excessive noise subtraction. Uniquely in ClariCT, the forward projection and FBP reconstruction steps are performed only using DICOM data; therefore, the entire hybrid IR is carried out in a vendor-neutral manner.
We thought that ClariCT may have the potential to provide enhanced denoising performance and radiation dose reduction as well as the convenience of vendor-neutral technique. Several previous studies investigated the performance of image-based IR technique and demonstrated similarity with vendor-supplied IR techniques (
27282930). However, those studies were conducted in adults; to date, there has been no investigation of image quality using vendor-neutral IR method in young patients.
Previous studies have focused on the comparison of vendor-specific and vendor-neutral IR techniques in low-dose CT (
27282930), or the performance evaluation of vendor-neutral IR using homogeneous lesions (
2730). Fletcher et al. (
27) reported non-inferiority between vendor-independent and vendor-specific IR methods in CT enterography. Other studies compared image qualities between vendor-specific and vendor-neutral methods (i.e., SafeCT) (
282930), acquiring both low- and standard-dose images, prospectively. In our study, the young patients underwent abdominal CT for various reasons, and the radiation doses varied significantly to balance the exposure and diagnostic performance. These parameters may affect the study design and its observations. However, in actual clinical practice, pediatric radiologists examine images of varying qualities depending on the patients' age, radiation dose, or image noise. Additionally, they are required to detect abnormalities of various organs. Evaluation of vendor-neutral IR techniques using multi-organ lesions and varying radiation doses might better represent the actual clinical setting and is advantageous to our study.
Because radiation reduction is a critical issue in young patients, previous studies evaluated the effect of IR at ultralow radiation dose levels with an anthropomorphic phantom (
910). However, in this study, phantom was not scanned with ultralow radiation dose (less than 0.5 mGy), because that level of radiation dose may not be used in clinical setting. Indeed, radiation dose was more than 0.5 mGy in our study population.
In terms of image noise reduction, ClariCT showed higher noise reduction power at a lower radiation dose level. This could be of clinical significance especially for young patients, because constant noise level is important for interpretation and is difficult to achieve in those patients due to high variations in body sizes and shapes. This might be attributable to differences in denoise processing between iDose and ClariCT, in which denoising effects on the sinogram and CT image could be combined differently depending on the confidence of noise separation power in each technique.
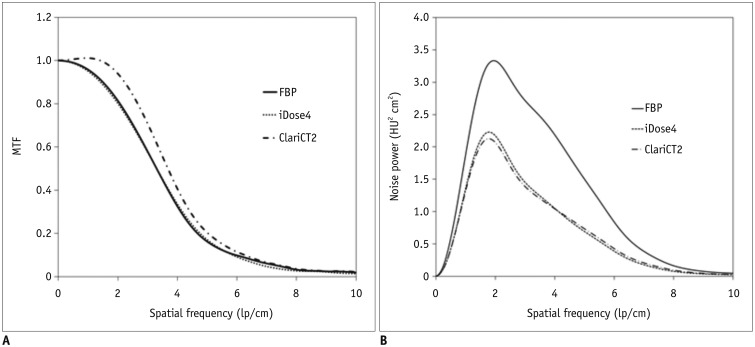
MTFs of FBP and iDose were almost identical, while that of ClariCT showed right shift at all spatial frequency ranges leading to a wider area under the curve. NPS curves of ClariCT and iDose showed a stronger downward shift, with a flatter overall shape in ClariCT than seen with iDose. Based on the interpretation of the frequency spectrum, a wider MTF curve represents better capability of preserving details of structural patterns whereas a flatter NPS curve is related to a finer image texture. Therefore, our data may indicate that ClariCT has superior capability than iDose in preserving fine details of structures as well as providing a finer image texture after denoise processing.
In our institute, vendor-provided denoising technique of level 4 (iDose4) has been used, as the images of that level have an acceptable noise level without significant image quality deterioration (
21). In the phantom study, absolute noise level of iDose4 was comparable to that of ClariCT2. Based on these phantom study results, ClariCT2 were selected for quality comparison of clinical imaging data.
Indeed, in the subjective analysis, ClariCT2 was comparable to iDose4 in terms of overall image quality and noise. ClariCT2 and iDose4 were also equivalent in artifact and lesion conspicuity scores. However, these two denoising techniques failed to show significant differences in artifact and lesion conspicuity compared with FBP, possibly due to heterogeneity of study population and lesions. The absence of differences in lesion conspicuity could be explained by the fact that evaluation of lesion conspicuity is highly dependent on personal experience and performance, and observer performance may not be directly linked to subjective image quality.
The strength of image-based noise reduction technique is vendor independency. Hence, simultaneous denoise processing of CT images from multiple vendors is possible and can be applied to images obtained with older CT systems for which modern vendor-provided denoising techniques are unavailable. The disadvantage of image-based denoising is loss of workflow efficiency, as FBP images are transferred to a server, denoised, and then transferred to PACS. In our test environment, the mean denoising time was 0.3 seconds per slice, and the overall transfer and processing of an exam resulted in a 2–3 minutes delay. In many practical applications, however, few minutes delay may not impair clinical workflow. In addition, the use of higher-end workstation may improve the workflow efficiency.
This study has several limitations. First, we evaluated vendor-neutral IR technique in only one type of CT equipment in clinically acceptable range of radiation doses (more than 0.5 mGy). Therefore, to demonstrate true vendor-neutrality of the technique, evaluation of ClariCT for CT equipment by other vendors is needed. In addition, we did not compare the performance of ClariCT with model-based IR techniques. However, in this study, we aimed to evaluate the clinical applicability of ClariCT, which showed more similarity to hybrid IR technique than model-based one, by comparing clinically used hybrid IR technique (i.e., iDose4). Therefore, comparison between ClariCT and other model-based IR technique or evaluation of denoising performance in ultra-low radiation dose (less than 0.5 mGy) might be required in these contexts. Second, due to limited clinical data, no image reconstruction was made at variable levels other than iDose4; hence, it was difficult to fully compare iDose with ClariCT at all levels. Therefore, we compared the image quality with various levels of reconstruction strength using a pediatric phantom. Third, the objective radiologist performance such as detectability of subtle lesions could not be measured, as those tasks cannot be performed in a fully blinded fashion. Finally, the evaluation of lesion conspicuity according to each disease entity could not be performed, due to the small sample size.
In conclusion, ClariCT is a vendor-neutral IR technique that shows similar image quality to clinically used vendor-specific hybrid IR in pediatric phantom and abdominopelvic CT in young patients. Additional validation studies using CT machines from other vendors are required.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download