Abstract
Pancreatic cancer is the ninth common malignancy in South Korea. It has a dismal prognosis with a 5-year overall survival rate of less than 10%, and pancreatic cancer is associated with cancer cachexia, which is defined as the loss of muscle mass that is not reversible by conventional nutritional support. Cachexia is noted in over 85% of all pancreatic cancer patients and it is strongly related with the disease’s mortality. Nearly 30% of pancreatic cancer deaths are due to cachexia rather than being due to the tumor burden. Therefore, it is crucial to discover the mechanisms behind the development of muscle wasting in pancreatic cancer patients and find novel therapeutics for targeting cachexia. This review deals with the current understanding about the development of cachexia and nutritional support in those patients suffering with pancreatic cancer.
Figures and Tables
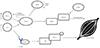 | Fig. 1Proinflammatory cytokines are drivers of cancer-induced cachexia. Proinflammatory cytokines IL-6 and TNF-α activate downstream signaling of NF-kB and this results in overstimulation of NF-kB and degradation of muscle proteins through the production of catabolic cytokines. Cytokines can also activate the Jak2/STAT3 signaling pathways that can lead to degradation of skeletal muscle protein. TNF-α, tumor necrosis factor α; MuRF1, muscle RING finger-containing protein 1; IL, interleukin. |
References
1. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013; 369:1691–1703.
2. Koutsounas I, Giaginis C, Patsouris E, Theocharis S. Current evidence for histone deacetylase inhibitors in pancreatic cancer. World J Gastroenterol. 2013; 19:813–828.

3. Kneuertz PJ, Cunningham SC, Cameron JL, et al. Palliative surgical management of patients with unresectable pancreatic adenocarcinoma: trends and lessons learned from a large, single institution experience. J Gastrointest Surg. 2011; 15:1917–1927.

4. Wigmore SJ, Plester CE, Richardson RA, Fearon KC. Changes in nutritional status associated with unresectable pancreatic cancer. Br J Cancer. 1997; 75:106–109.

5. Gärtner S, Krüger J, Aghdassi AA, et al. Nutrition in pancreatic cancer: a review. Gastrointest Tumors. 2016; 2:195–202.

6. Fearon KC, Baracos VE. Cachexia in pancreatic cancer: new treatment options and measures of success. HPB (Oxford). 2010; 12:323–324.

7. Kyle UG, Pirlich M, Lochs H, Schuetz T, Pichard C. Increased length of hospital stay in underweight and overweight patients at hospital admission: a controlled population study. Clin Nutr. 2005; 24:133–142.

8. Bachmann J, Heiligensetzer M, Krakowski-Roosen H, Büchler MW, Friess H, Martignoni ME. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg. 2008; 12:1193–1201.

10. Bachmann J, Ketterer K, Marsch C, et al. Pancreatic cancer related cachexia: influence on metabolism and correlation to weight loss and pulmonary function. BMC Cancer. 2009; 9:255.

11. Felix K, Fakelman F, Hartmann D, et al. Identification of serum proteins involved in pancreatic cancer cachexia. Life Sci. 2011; 88:218–225.

12. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011; 12:489–495.

13. Skipworth RJ, Stewart GD, Dejong CH, Preston T, Fearon KC. Pathophysiology of cancer cachexia: much more than host-tumour interaction? Clin Nutr. 2007; 26:667–676.

14. Stewart GD, Skipworth RJ, Fearon KC. Cancer cachexia and fatigue. Clin Med (Lond). 2006; 6:140–143.

15. Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci. 1997; 52:B267–B276.

16. Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012; 16:153–166.

17. Mace TA, Ameen Z, Collins A, et al. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013; 73:3007–3018.

18. Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol. 2015; 22:100–106.

19. Martignoni ME, Kunze P, Hildebrandt W, et al. Role of mononuclear cells and inflammatory cytokines in pancreatic cancer-related cachexia. Clin Cancer Res. 2005; 11:5802–5808.

20. Denley SM, Jamieson NB, McCall P, et al. Activation of the IL-6R/Jak/stat pathway is associated with a poor outcome in resected pancreatic ductal adenocarcinoma. J Gastrointest Surg. 2013; 17:887–898.

21. Mitsunaga S, Ikeda M, Shimizu S, et al. Serum levels of IL-6 and IL-1β can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. 2013; 108:2063–2069.

22. Pop VV, Seicean A, Lupan I, Samasca G, Burz CC. IL-6 roles - molecular pathway and clinical implication in pancreatic cancer - a systemic review. Immunol Lett. 2017; 181:45–50.

23. Farren MR, Mace TA, Geyer S, et al. Systemic immune activity predicts overall survival in treatment-naïve patients with metastatic pancreatic cancer. Clin Cancer Res. 2016; 22:2565–2574.

24. Zhou W, Jiang ZW, Tian J, Jiang J, Li N, Li JS. Role of NF-kappaB and cytokine in experimental cancer cachexia. World J Gastroenterol. 2003; 9:1567–1570.
25. Bonetto A, Aydogdu T, Jin X, et al. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab. 2012; 303:E410–E421.

26. Bonetto A, Aydogdu T, Kunzevitzky N, et al. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS One. 2011; 6:e22538.

27. Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS Jr. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000; 289:2363–2366.
28. Zhang L, Tang H, Kou Y, et al. MG132-mediated inhibition of the ubiquitin-proteasome pathway ameliorates cancer cachexia. J Cancer Res Clin Oncol. 2013; 139:1105–1115.

29. Melstrom LG, Melstrom KA Jr, Ding XZ, Adrian TE. Mechanisms of skeletal muscle degradation and its therapy in cancer cachexia. Histol Histopathol. 2007; 22:805–814.
30. Fogelman DR, Morris J, Xiao L, et al. A predictive model of inflammatory markers and patient-reported symptoms for cachexia in newly diagnosed pancreatic cancer patients. Support Care Cancer. 2017; 25:1809–1817.

31. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997; 387:83–90.
32. Chen YG, Wang Q, Lin SL, Chang CD, Chuang J, Ying SY. Activin signaling and its role in regulation of cell proliferation, apoptosis, and carcinogenesis. Exp Biol Med (Maywood). 2006; 231:534–544.

33. Sartori R, Milan G, Patron M, et al. Smad2 and 3 transcription factors control muscle mass in adulthood. Am J Physiol Cell Physiol. 2009; 296:C1248–C1257.

34. Loumaye A, de Barsy M, Nachit M, et al. Role of activin A and myostatin in human cancer cachexia. J Clin Endocrinol Metab. 2015; 100:2030–2038.

35. Zhou X, Wang JL, Lu J, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010; 142:531–543.

36. Togashi Y, Kogita A, Sakamoto H, et al. Activin signal promotes cancer progression and is involved in cachexia in a subset of pancreatic cancer. Cancer Lett. 2015; 356(2 Pt B):819–827.

37. Greco SH, Tomkötter L, Vahle AK, et al. TGF-β blockade reduces mortality and metabolic changes in a validated murine model of pancreatic cancer cachexia. PLoS One. 2015; 10:e0132786.

38. Miyamoto Y, Hanna DL, Zhang W, Baba H, Lenz HJ. Molecular pathways: cachexia signaling-a targeted approach to cancer treatment. Clin Cancer Res. 2016; 22:3999–4004.

40. Schmitt TL, Martignoni ME, Bachmann J, et al. Activity of the Akt-dependent anabolic and catabolic pathways in muscle and liver samples in cancer-related cachexia. J Mol Med (Berl). 2007; 85:647–654.

41. Avan A, Avan A, Le Large TY, et al. AKT1 and SELP polymorphisms predict the risk of developing cachexia in pancreatic cancer patients. PLoS One. 2014; 9:e108057.

42. Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab. 2004; 287:E591–E601.

43. Abbott CR, Monteiro M, Small CJ, et al. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005; 1044:127–131.

44. Kim SY, Park SM, Lee ST. Apolipoprotein C-II is a novel substrate for matrix metalloproteinases. Biochem Biophys Res Commun. 2006; 339:47–54.

45. Russell ST, Zimmerman TP, Domin BA, Tisdale MJ. Induction of lipolysis in vitro and loss of body fat in vivo by zinc-alpha2-glycoprotein. Biochim Biophys Acta. 2004; 1636:59–68.
46. Ronga I, Gallucci F, Riccardi F, Uomo G. Anorexia-cachexia syndrome in pancreatic cancer: recent advances and new pharmacological approach. Adv Med Sci. 2014; 59:1–6.

47. Uomo G, Gallucci F, Rabitti PG. Anorexia-cachexia syndrome in pancreatic cancer: recent development in research and management. JOP. 2006; 7:157–162.
48. Ramos EJ, Suzuki S, Marks D, Inui A, Asakawa A, Meguid MM. Cancer anorexia-cachexia syndrome: cytokines and neuropeptides. Curr Opin Clin Nutr Metab Care. 2004; 7:427–434.

49. Mueller TC, Burmeister MA, Bachmann J, Martignoni ME. Cachexia and pancreatic cancer: are there treatment options? World J Gastroenterol. 2014; 20:9361–9373.
50. Sikkens EC, Cahen DL, de Wit J, Looman CW, van Eijck C, Bruno MJ. Prospective assessment of the influence of pancreatic cancer resection on exocrine pancreatic function. Br J Surg. 2014; 101:109–113.

51. Hackert T, Schütte K, Malfertheiner P. The pancreas: causes for malabsorption. Viszeralmedizin. 2014; 30:190–197.

52. Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011; 98:268–274.

53. Loh KW, Vriens MR, Gerritsen A, et al. Unintentional weight loss is the most important indicator of malnutrition among surgical cancer patients. Neth J Med. 2012; 70:365–369.
54. La Torre M, Ziparo V, Nigri G, Cavallini M, Balducci G, Ramacciato G. Malnutrition and pancreatic surgery: prevalence and outcomes. J Surg Oncol. 2013; 107:702–708.

55. Billingsley KG, Hur K, Henderson WG, Daley J, Khuri SF, Bell RH Jr. Outcome after pancreaticoduodenectomy for periampullary cancer: an analysis from the veterans affairs national surgical quality improvement program. J Gastrointest Surg. 2003; 7:484–491.

56. Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the national VA surgical risk study. Arch Surg. 1999; 134:36–42.
57. Augustin T, Burstein MD, Schneider EB, et al. Frailty predicts risk of life-threatening complications and mortality after pancreatic resections. Surgery. 2016; 160:987–996.

58. Imaoka H, Mizuno N, Hara K, et al. Evaluation of modified glasgow prognostic score for pancreatic cancer: a retrospective cohort study. Pancreas. 2016; 45:211–217.
59. Lucas DJ, Schexneider KI, Weiss M, et al. Trends and risk factors for transfusion in hepatopancreatobiliary surgery. J Gastrointest Surg. 2014; 18:719–728.

60. Sikkens EC, Cahen DL, de Wit J, Looman CW, van Eijck C, Bruno MJ. A prospective assessment of the natural course of the exocrine pancreatic function in patients with a pancreatic head tumor. J Clin Gastroenterol. 2014; 48:e43–e46.

61. Lindkvist B, Phillips ME, Domínguez-Muñoz JE. Clinical, anthropometric and laboratory nutritional markers of pancreatic exocrine insufficiency: prevalence and diagnostic use. Pancreatology. 2015; 15:589–597.

62. Roeyen G, Jansen M, Chapelle T, et al. Diabetes mellitus and pre-diabetes are frequently undiagnosed and underreported in patients referred for pancreatic surgery. A prospective observational study. Pancreatology. 2016; 16:671–676.

63. Illés D, Terzin V, Holzinger G, et al. New-onset type 2 diabetes mellitus--a high-risk group suitable for the screening of pancreatic cancer? Pancreatology. 2016; 16:266–271.
64. Gupta S, Vittinghoff E, Bertenthal D, et al. New-onset diabetes and pancreatic cancer. Clin Gastroenterol Hepatol. 2006; 4:1366–1372.

65. Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008; 134:981–987.

66. Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007; 132:2208–2225.

67. Hart PA, Baichoo E, Bi Y, Hinton A, Kudva YC, Chari ST. Pancreatic polypeptide response to a mixed meal is blunted in pancreatic head cancer associated with diabetes mellitus. Pancreatology. 2015; 15:162–166.

68. Cooperman AM, Chivati J, Chamberlain RS. Nutritional and metabolic aspects of pancreatic cancer. Curr Opin Clin Nutr Metab Care. 2000; 3:17–21.

69. Klek S, Sierzega M, Szybinski P, et al. The immunomodulating enteral nutrition in malnourished surgical patients - a prospective, randomized, double-blind clinical trial. Clin Nutr. 2011; 30:282–288.

70. Gianotti L, Braga M, Gentilini O, Balzano G, Zerbi A, Di Carlo V. Artificial nutrition after pancreaticoduodenectomy. Pancreas. 2000; 21:344–351.

71. Di Carlo V, Gianotti L, Balzano G, Zerbi A, Braga M. Complications of pancreatic surgery and the role of perioperative nutrition. Dig Surg. 1999; 16:320–326.

72. Karagianni VT, Papalois AE, Triantafillidis JK. Nutritional status and nutritional support before and after pancreatectomy for pancreatic cancer and chronic pancreatitis. Indian J Surg Oncol. 2012; 3:348–359.

73. Park JS, Chung HK, Hwang HK, Kim JK, Yoon DS. Postoperative nutritional effects of early enteral feeding compared with total parental nutrition in pancreaticoduodectomy patients: a prosepective, randomized study. J Korean Med Sci. 2012; 27:261–267.

74. Liu C, Du Z, Lou C, et al. Enteral nutrition is superior to total parenteral nutrition for pancreatic cancer patients who underwent pancreaticoduodenectomy. Asia Pac J Clin Nutr. 2011; 20:154–160.
75. Park JW, Jang JY, Kim EJ, et al. Effects of pancreatectomy on nutritional state, pancreatic function and quality of life. Br J Surg. 2013; 100:1064–1070.

76. Nussbaum DP, Zani S, Penne K, et al. Feeding jejunostomy tube placement in patients undergoing pancreaticoduodenectomy: an ongoing dilemma. J Gastrointest Surg. 2014; 18:1752–1759.

77. Richter E, Denecke A, Klapdor S, Klapdor R. Parenteral nutrition support for patients with pancreatic cancer--improvement of the nutritional status and the therapeutic outcome. Anticancer Res. 2012; 32:2111–2118.
78. Pelzer U, Arnold D, Gövercin M, et al. Parenteral nutrition support for patients with pancreatic cancer. Results of a phase II study. BMC Cancer. 2010; 10:86.

79. Vashi PG, Dahlk S, Popiel B, Lammersfeld CA, Ireton-Jones C, Gupta D. A longitudinal study investigating quality of life and nutritional outcomes in advanced cancer patients receiving home parenteral nutrition. BMC Cancer. 2014; 14:593.

80. Davidson W, Ash S, Capra S, Bauer J. Cancer Cachexia Study Group. Weight stabilisation is associated with improved survival duration and quality of life in unresectable pancreatic cancer. Clin Nutr. 2004; 23:239–247.

81. Wigmore SJ, Barber MD, Ross JA, Tisdale MJ, Fearon KC. Effect of oral eicosapentaenoic acid on weight loss in patients with pancreatic cancer. Nutr Cancer. 2000; 36:177–184.

82. Fearon KC, Von Meyenfeldt MF, Moses AG, et al. Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut. 2003; 52:1479–1486.

83. Heller AR, Rössel T, Gottschlich B, et al. Omega-3 fatty acids improve liver and pancreas function in postoperative cancer patients. Int J Cancer. 2004; 111:611–616.

84. Arshad A, Isherwood J, Mann C, et al. Intravenous ω-3 fatty acids plus gemcitabine. JPEN J Parenter Enteral Nutr. 2017; 41:398–403.

85. Kraft M, Kraft K, Gärtner S, et al. L-carnitine-supplementation in advanced pancreatic cancer (CARPAN)--a randomized multi-centre trial. Nutr J. 2012; 11:52.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download