This article has been
cited by other articles in ScienceCentral.
Abstract
Background and Objectives
Left ventricular (LV) apical thrombi are usually present with LV dilatation, and oral anticoagulants reduce embolic risk in these patients. However, echocardiographic data regarding thrombus resolution remain limited. We studied its echocardiographic features that were associated with early resolution (within 1 month).
Methods
We performed a retrospective observational study by reviewing baseline and follow-up echocardiographic images and medical records in patients with LV apical thrombi.
Results
Between January 2005 and December 2017, 77 patients (59 males, mean 61±12 years old) were enrolled. Patients were classified into 2 groups based on duration of thrombus resolution: group 1 showing resolution within 1 month (n=23) and group 2 with persistence after 1 month (n=54). Thrombus size was significantly smaller in group 1 (10.7±4.2 vs. 12.1±5.5 mm, p=0.046). Grade 1 mobility (partially mobile; odds ratio [OR], 7.800; p=0.012) and grade 2 mobility (highly mobile; OR, 14.625; p=0.002) were significantly associated with the early resolution. Round thrombi were associated with early resolution than mural form (OR, 3.187; p=0.026). Multivariate analysis showed that the mobility was the most important parameter, and a highly mobile (grade 2 mobility) LV apical thrombi showed earlier resolution (OR, 12.525; p=0.013). During the follow-up over 62±44 months, 25 patients (32.5%) had ≥1 adverse clinical events. The late resolution of thrombi was associated with poor long-term clinical outcomes (hazard ratio, 5.727; p=0.020).
Conclusions
Mobility of LV apical thrombi was the most important parameter associated with early thrombus resolution. Late resolution of LV apical thrombi was associated with poor long-term clinical outcomes.
Keywords: Thrombus, Left ventricle, Anticoagulants, Embolism
INTRODUCTION
Left ventricular (LV) apical thrombi are usually formed in a dilated LV with conditions that favor blood stasis, increased coagulability and endothelial injury.
1) These conditions include acute myocardial infarction (MI) and non-ischemic cardiomyopathies. Once formed, LV apical thrombi can be associated with increased risk of thromboembolic events such as stroke.
2)3) Management of LV apical thrombi primarily involves anticoagulation therapy using oral vitamin K antagonists. The European Society of Cardiology guidelines recommend the administration of anticoagulants using vitamin K antagonists over 6 months based on repeated imaging studies.
4) Anticoagulant therapy reduces the risk of embolic events associated with LV apical thrombus.
5) The echocardiographic examination is the most commonly used imaging modality that helps the identification and follow-up of LV apical thrombi. However, there is limited echocardiographic data available regarding the time required for resolution of LV apical thrombi after the detection and initiation of anticoagulant therapy. Thus, we aimed to identify the echocardiographic features of LV apical thrombi that were associated with early resolution (within 1 month).
METHODS
Study population and study protocol
We performed a retrospective, single-center observational study at the Chungnam National University Hospital, Korea. We screened all consecutive patients with LV apical thrombi during echocardiographic examinations between January 2005 and December 2017. We included patients with follow-up echocardiographic examinations within 1 month. We excluded patients without follow-up echocardiographic data within 1 month, patients who did not receive anticoagulants, and those who underwent surgical removal of a thrombus at the time of a coronary artery bypass graft surgery. Details pertaining to the demographic, clinical, and angiographic data of patients were obtained from electronic medical records.
Echocardiographic measurement
Two independent echocardiography specialists (JK Oh and JH Park) re-analyzed all digitally stored echocardiographic images at baseline, at the time of diagnosis, and at the time of follow-up echocardiographic examinations. Two-dimensional and Doppler echocardiographic examinations were performed by experienced sonographers using commercially available equipment with standardized techniques.
The LV ejection fraction (LVEF) was calculated using the biplane modified Simpson's method, and LV diastolic filling patterns were assessed using a pulsed-wave Doppler tracing of the mitral inflow including mitral E and A wave velocity. Mitral annular velocities were measured using analysis of pulsed-wave tissue-Doppler images. The LV end-diastolic pressure was estimated using the ratio of mitral E wave velocity to mitral annular E′ wave velocity.
An LV apical aneurysm was defined as an obvious outward bulging of the LV apical wall during diastole with a lack of wall motion (akinesia) observed during systole or a paradoxical expansion (dyskinesia).
6) The extent of an LV apical aneurysm is expressed both as a ratio of circumferential length and as a ratio of circumferential area of the aneurysm to the LV in end-diastole in apical 4-chamber view.
An apical LV apical thrombus was diagnosed when an echo-dense mass with clearly distinct margin from the LV endocardium was identified using at least 2 echocardiographic views and was observed to be attached to the LV apical wall.
7) Characteristics of a thrombus were described in terms of the following: the shape of a thrombus was classified as ‘mural’ or ‘round’. A ‘mural thrombus’ was defined as one with a free concave margin that followed the curvature of the LV wall. A ‘round thrombus’ was defined as one that was spherical and projected into the LV cavity. The maximal thrombus size was measured perpendicular to the myocardium from the endocardial border extending to the innermost border of the thrombus-blood interface when viewed using apical views. A mobile thrombus was defined as one whose motion was independent of the motion of the adjacent LV wall segment. The degree of mobility was graded as 0 (fixed thrombus), 1 (partially mobile), and 2 (highly mobile) based on visual assessment of real-time images.
8) A highly mobile thrombus was defined as one showing vigorous motion of the intra-cavitary portion of the thrombus or eccentric motion of fluffy and wavy strands. The presence of a central halo was defined as a thrombus showing a lower density in its inner segment than in the area around it.
Clinical profiles and follow-up
Baseline demographic data and past clinical history were obtained from patients' medical records. Diabetic patients were defined as those receiving active treatment with oral hypoglycemic agents or insulin. A diagnosis of diabetes was also confirmed in patients showing an abnormal fasting blood glucose level (≥126 mg/dL) or an abnormal blood glucose level 2 hours after meals (≥200 mg/dL) in those treated only with dietary modification. Hypertension was defined as the use of antihypertensive medication for >6 months. We also confirmed the presence of hypertension in patients who were diagnosed with hypertension and treated only with lifestyle modifications. Dyslipidemia was defined as the use of cholesterol-lowering agents in patients or those showing abnormal serum cholesterol levels (≥200 mg/dL) upon admission. Chronic kidney disease was defined in patients with an estimated glomerular filtration rate <30 mL/min/1.73m2 or in patients with hemodialysis. Dilated cardiomyopathy was diagnosed in patients without obvious causes causing heart failure. It was defined with the presence of globular LV with LV end-diastolic internal dimension >56 mm, LV sphericity index ratio of less than 1.5, and LVEF<40%. Patients were followed over 62±44 months to monitor for occurrences of adverse clinical events including death and hospitalization for heart failure or stroke. The occurrence of clinical events was determined through the review of patients' medical records in those who received regular clinical follow-ups. In patients without regular clinical follow-up, death of a patient was confirmed by checking with a nation-wide database maintained by the National Health Institute Service in Korea. The study protocol has been reviewed and approved by the Institutional Review Board (IRB) of Chungnam National University Hospital (IRB No. 2018-03-061), and conducted according to the principles of the Declaration of Helsinki. The IRB waved the need for written informed consent from the study patients.
Statistical analysis
Patients' characteristics were presented using mean±standard deviation for continuous data and frequencies and percentages for categorical data. We compared categorical variables using the χ2 test and continuous variables using the independent samples t-test. We used a binary logistic regression model to determine the association between the studied variables and LV apical thrombus resolution. Odds ratios (ORs) have been shown with their 95% confidence intervals (CIs). The OR refers to a unit increase in the variables. Factors that showed statistical significance using univariate analysis were subjected to multivariate analysis. To exclude an over-fit of the analysis model, we selected up to 3 variables for this analysis. A Cox proportional hazards model was used to assess the time to first adverse clinical event. Hazard ratios (HRs) were given with the 95% CI. A 2-tailed p value<0.05 was considered statistically significant. All statistical analyses were performed using the SPSS software, version 20.0 (IBM, Armonk, NY, USA).
RESULTS
Between January 2005 and December 2017, we screened a total of 132 patients with a diagnosis of an LV apical thrombus. Among these, 55 patients were excluded for various reasons, and 77 patients (59 men, mean age 61±12 years) were eventually enrolled in our study (
Figure 1). Follow-up echocardiography was performed serially on days 14 and 30 after the detection of LV apical thrombus to confirm the resolution of thrombus. Seventy-one patients (92%) were performed within 14 days, and the others were performed follow-up echocardiography within 30 days. The next follow-up echocardiographic examinations after 1 month were done by attending physician's decision. Thrombi showed resolution within 14 days in 16 patients (20.8%), between 14 days and 1 month in 7 patients (9.0%) and 1 month later in 46 patients (59.7%). However, 8 patients did not show complete resolution using anticoagulant therapy.
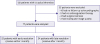 | Figure 1 Flow diagram of this study.
|
Anticoagulants were administered after the identification of an LV apical thrombus in patients without contraindication for oral anticoagulant medication. Oral anticoagulant therapy was initiated and continued following the administration of 5-day intravenous bolus heparin. Warfarin was the most commonly used oral anticoagulant, which was prescribed in 70 patients (88%). Novel oral anticoagulant agents such as direct factor Xa inhibitors were administrated to 5 patients (6%), and unfractionated heparin (UFH) or low molecular weight heparin (LMWH) were continuously administered until complete resolution of the thrombus to 2 patients (3%) who were scheduled for major surgery. There was no difference of international normalization ratio between 2 groups within 1 months (2.5±0.5 vs. 2.6±0.4, p=0.449). Further duration of oral anticoagulation therapy was determined by physician's decision.
LV apical thrombi were resolved in a median period of 63 days (interquartile range, 29–158 days) after their identification. Most patients showed thrombus resolution within 6 months, but a persistent thrombus was observed in 8 patients who continued anticoagulants even after 1 year. Based on the duration of thrombus resolution, patients were classified into 2 groups: the early resolution group (group 1), in which patients showed resolution of an apical LV apical thrombus within 1 month, and the late resolution group (group 2) in which patients had an LV apical thrombus even after 1 month. Baseline and echocardiographic findings were summarized in
Table 1. In terms of baseline clinical characteristics, females were more frequent in the group 1 than in the group 2 (39.1% vs. 17.7%, p=0.043). Moreover, female sex was a significant predictor of the early thrombus resolution (OR, 3.214; 95% CI, 1.068–9.671; p=0.038). Dyslipidemia was significantly predominant in the group 2 (4.3% vs. 33.3%, p=0.008), and patients with previous MI were more numerous in group 2 (0% vs. 33%, p=0.001). LV apical thrombus occurred in 56 patients with ischemic cardiomyopathy, and in 21 patients with non-ischemic cardiomyopathy. Non-ischemic cardiomyopathy included both dilated cardiomyopathy and stress-induced cardiomyopathy. There was no significant difference in the proportion of the etiology of LV systolic dysfunction (p=0.405). There was no significant difference of the systolic and diastolic LV diameters (p=0.248 and p=0.258) and LVEF (p=0.775). There was an insignificant difference of diastolic parameters between the 2 groups.
Table 1
Baseline clinical and echocardiographic parameters associated with early resolution of an LV apical thrombus

|
Variable |
Independent sample t-test |
Logistic regression analysis |
|
Total (n=77) |
Group 1 (n=23) |
Group 2 (n=54) |
p value |
OR (95% CI)*
|
p value |
|
Age (years) |
60.8±12.3 |
61.6±12.7 |
60.5±12.2 |
0.739 |
1.007 (0.967–1.040) |
0.735 |
|
Female (sex) |
18 (23.4) |
9 (39.1) |
9 (17.7) |
0.043 |
3.214 (1.068–9.671) |
0.038 |
|
BMI (kg/m2) |
23.9±3.9 |
22.7±3.5 |
24.3±4.0 |
0.125 |
0.891 (0.768–1.034) |
0.129 |
|
Previous MI |
18 (23.4) |
0 |
18 (33.3) |
0.001 |
- |
- |
|
Atrial fibrillation |
4 (5.2) |
1 (4.3) |
3 (5.6) |
1.000 |
0.773 (0.076–7.845) |
0.773 |
|
Cardiovascular risk factor |
|
|
|
|
|
|
|
Hypertension |
30 (39.0) |
9 (39.1) |
21 (38.9) |
1.000 |
1.010 (0.372–2.747) |
0.984 |
|
Diabetes |
20 (26.0) |
6 (26.1) |
14 (25.9) |
1.000 |
1.008 (0.332–3.066) |
0.988 |
|
Dyslipidemia |
19 (24.7) |
1 (4.3) |
18 (33.3) |
0.008 |
0.091 (0.011–0.729) |
0.024 |
|
CKD |
6 (7.8) |
2 (8.7) |
4 (7.4) |
1.000 |
1.190 (0.202–7.005) |
0.847 |
|
Etiology |
|
|
|
0.405 |
|
|
|
Ischemic cardiomyopathy |
56 (72.7) |
15 (65.2) |
41 (75.9) |
0.596 (0.206–1.717) |
0.337 |
|
Non-ischemic cardiomyopathy |
21 (27.3) |
13 (24.1) |
8 (34.8) |
Reference |
|
|
Baseline echocardiographic findings |
|
|
|
|
|
|
|
LVIDs (mm) |
44.5±11.9 |
42.1±13.2 |
45.6±11.2 |
0.248 |
0.975 (0.934–1.018) |
0.246 |
|
LVIDd (mm) |
54.3±8.7 |
52.6±9.9 |
55.1±8.2 |
0.258 |
0.966 (0.911–1.025) |
0.256 |
|
LVEF |
32.3±12.4 |
32.9±14.3 |
32.0±11.6 |
0.775 |
1.006 (0.967–1.047) |
0.772 |
|
LVGLS |
−4.8±2.7 |
−5.3±2.8 |
−4.4±2.7 |
0.227 |
0.889 (0.734–1.076) |
0.227 |
|
Mitral E velocity (cm/s) |
71.8±23.5 |
69.3±20.6 |
72.8±24.8 |
0.571 |
0.993 (0.971–1.016) |
0.565 |
|
Mitral A velocity (cm/s) |
62.9±28.9 |
68.0±24.1 |
60.7±30.8 |
0.353 |
1.009 (0.990–1.027) |
0.352 |
|
Mitral DT (ms) |
161.9±64.8 |
165.3±49.7 |
160.5±70.5 |
0.785 |
1.001 (0.993–1.009) |
0.782 |
|
Mitral E/A ratio |
1.5±1.2 |
1.3±1.2 |
1.6±1.2 |
0.380 |
0.793 (0.473–1.328) |
0.378 |
|
Mitral annular E′ velocity (cm/s) |
4.8±1.8 |
4.8±1.6 |
4.8±1.8 |
0.997 |
0.999 (0.732–1.364) |
0.997 |
|
Mitral E/E′ ratio |
17.2±8.9 |
16.3±8.0 |
17.6±9.4 |
0.600 |
0.983 (0.923–1.047) |
0.594 |
|
SEC in LV |
30 (39.0) |
9 (39.1) |
21 (38.9) |
1.000 |
1.010 (0.372–2.747) |
0.984 |
|
LV apical aneurysm |
57 (74.0) |
16 (69.6) |
41 (75.9) |
0.580 |
0.725 (0.245–2.146) |
0.561 |
|
LV aneurysm, height (cm) |
2.6±1.8 |
2.1±1.6 |
2.7±1.9 |
0.160 |
0.818 (0.618–1.083) |
0.160 |
|
LV aneurysm |
38.6±12.7 |
35.1±11.6 |
40.0±13.0 |
0.201 |
0.969 (0.923–1.017) |
0.201 |
|
Thrombus size, transverse (mm) |
11.7±5.2 |
16.8±6.4 |
21.4±10.2 |
0.295 |
0.945 (0.851–1.050) |
0.294 |
|
Thrombus size, longitudinal (mm) |
20.0±9.4 |
10.7±4.2 |
12.1±5.5 |
0.046 |
0.938 (0.878–1.001) |
0.053 |
|
Grade of mobility |
|
|
|
0.002 |
|
0.009 |
|
|
0 (no mobility) |
28 (36.4) |
2 (8.7) |
26 (48.1) |
Reference |
|
|
|
1 (partial mobility) |
32 (41.6) |
12 (52.2) |
20 (37.0) |
7.800 (1.565–38.884) |
0.012 |
|
|
2 (high mobility) |
17 (22.1) |
9 (39.1) |
8 (14.8) |
14.625 (2.606–82.080) |
0.002 |
|
Shape |
|
|
|
0.027 |
|
|
|
|
Mural thrombus |
42 (54.5) |
8 (34.8) |
34 (63.0) |
Reference |
|
|
|
Round thrombus |
35 (45.5) |
15 (68.2) |
20 (36.4) |
3.187 (1.149–8.844) |
0.026 |
|
Halo in thrombus |
48 (62.3) |
14 (60.9) |
34 (63.0) |
1.000 |
0.915 (0.336–2.495) |
0.862 |
|
Follow-up echocardiographic findings at 1 month |
|
|
|
|
|
|
|
LVIDs (mm) |
42.2±11.3 |
40.3±15.8 |
43.8±10.3 |
0.403 |
0.974 (0.928–1.023) |
0.296 |
|
LVIDd (mm) |
53.5±7.7 |
51.4±10.8 |
54.9±7.5 |
0.148 |
0.950 (0.886–1.019) |
0.151 |
|
LVEF |
37.7±12.6 |
23.4±15.7 |
35.9±11.7 |
0.500 |
1.015 (0.977–1.055) |
0.442 |
|
Change of LVEF |
4.0±9.1 |
5.5±6.7 |
3.4±10.0 |
0.365 |
1.022 (0.966–1.081) |
0.448 |
|
Mitral E/E′ ratio |
15.1±7.9 |
14.4±6.6 |
15.3±8.2 |
0.716 |
0.983 (0.900–1.075) |
0.711 |
|
SEC in LV |
25 (32.5) |
6 (26.1) |
19 (35.2) |
0.596 |
0.650 (0.220–1.925) |
0.437 |
|
Medication use |
|
|
|
|
|
|
|
Warfarin |
67 (87.0) |
19 (82.6) |
48 (88.9) |
0.474 |
0.594 (0.151–2.342) |
0.457 |
|
Direct factor Xa inhibitor |
6 (7.9) |
1 (4.3) |
5 (9.4) |
0.661 |
0.436 (0.048–3.960) |
0.461 |
|
Maintain adequate anticoagulation |
59 (76.6) |
18 (78.3) |
41 (75.9) |
1.000 |
1.506 (0.433–5.234) |
0.519 |

The height (2.1±1.6 cm vs. 2.7±1.9 cm, p=0.160) and percentage of LV apical aneurysms (35.1±11.6% vs. 40.0±13.0%, p=0.201) were comparable between 2 groups. Thrombus size was significantly smaller in the group 1 than in the group 2 (10.7±4.2 mm vs. 12.1±5.5 mm, p=0.046) and a 1 mm increase in thrombus size tended to be associated with 6.2% slower resolution of an LV apical thrombus (OR, 0.938; 95% CI, 0.878–1.001; p=0.053). A statistically significant association was observed between the mobility and the early resolution. Grade 1 mobility (partially mobile; OR, 7.800; 95% CI, 1.565–38.884; p=0.019), and grade 2 mobility (highly mobile) were strongly associated with the early thrombus resolution (OR, 14.625; 95% CI, 2.606–82.080; p=0.002). Round thrombi showed earlier resolution than mural thrombi (OR, 3.187; 95% CI, 1.149–8.884; p=0.026).
Multivariate analysis showed that the mobility of the thrombus was the most important predictor of early thrombus resolution (
Table 2). Highly mobile LV apical thrombi were associated with early resolution (adjusted OR, 12.525; 95% CI, 1.720–91.206; p=0.013).
Table 2
Multivariate analysis in the prediction of early resolution of LV thrombus

|
Variable |
OR*
|
95% CI |
p value |
|
Female (sex) |
2.380 |
0.687–8.244 |
0.171 |
|
Thrombus size, longitudinal (mm) |
0.937 |
0.866–1.013 |
0.101 |
|
Grade of mobility |
|
|
0.036 |
|
0 (no mobility) |
Reference |
|
|
|
1 (partial mobility) |
7.105 |
1.317–38.339 |
0.023 |
|
2 (high mobility) |
12.525 |
1.720–91.206 |
0.013 |
|
Round thrombus |
1.057 |
0.283–3.944 |
0.934 |

During the follow-up over 62±44 months, 25 patients (32.5%) had more than 1 episode of adverse clinical events (15 deaths, 12 patients requiring hospitalization for heart failure, and 4 patients with strokes;
Table 3). The incidence of symptomatic stroke was 5.2%; 1 patient with a highly mobile thrombus developed a stroke within 4 days of initiation of anticoagulant therapy, and 3 of these patients who developed stroke belonged to group 2. Late resolution of an LV apical thrombus was associated with poor long-term clinical outcomes (HR, 5.727; 95% CI, 1.315–24.932; p=0.020;
Table 3).
Table 3
Long-term clinical outcomes according to the presence of early resolution of LV apical thrombus within 1 month

|
Clinical outcomes |
Independent sample t-test |
Univariate |
|
Group 1 (n=23) |
Group 2 (n=54) |
HR (95% CI)*
|
p value |
|
Stroke |
1 (4.3) |
3 (5.6) |
1.259 (0.131–12.133) |
0.842 |
|
All-cause mortality |
3 (13.0) |
12 (22.6) |
7.675 (0.965–61.045) |
0.054 |
|
Heart failure admission |
2 (8.7) |
10 (18.5) |
42.998 (0.168–10,974.517) |
0.183 |
|
Composite outcomes†
|
4 (17.4) |
21 (38.9) |
5.727 (1.315–24.932) |
0.020 |

DISCUSSION
This study showed that the mobility of an LV apical thrombus was the most important predictor of early thrombus resolution (within 1 month) and that late resolution (after 1 month) was associated with poor long-term clinical outcomes.
LV apical thrombus formation is known to occur in patients with acute anterior MI and dilated cardiomyopathy.
9) Reportedly, the incidence of LV apical thrombi was approximately 60% in patients with acute anterior MI particularly in the pre-thrombolytic era.
7)10) The formation of an LV apical thrombus was associated with reduced LVEF (≤35%) and presence of apical aneurysms.
9) Recently, percutaneous coronary intervention has replaced thrombolytic therapy as an effective means of decreasing the incidence of LV apical thrombi. Our previous study showed that the incidence of LV apical thrombi was 3.3% (34 of 1,045) in patients with acute anterior MI, and it was associated with impaired diastolic function.
11)
Because patients with an LV apical thrombus are at an increased risk of developing embolic events, early detection is important to reduce embolic potential with prompt preventive anticoagulation therapy. Echocardiographic examination is the most commonly used imaging study for the identification of LV apical thrombi.
12)13) The presence of an LV apical thrombus is associated with poor clinical outcomes.
14) The administration of anticoagulants reduce the risk of embolic events associated with LV apical thrombi in these patients.
5)15) Oral vitamin K antagonists are recommended concomitant with intravenous administration of UFH or subcutaneous LMWH in patients with LV apical thrombi.
4) The use of anticoagulants led to total thrombus resolution in 69 patients (89.6%) in our study. The remaining 8 patients had residual thrombi even after receiving anticoagulants for >1 year.
In addition to defining the characteristics of thrombi, echocardiographic studies provide additional information regarding the presence of LV apical aneurysms, spontaneous echo-contrast (SEC), and LVEF. These parameters are useful predictors of thrombus resolution. However, in this study, we included only the patients with LV apical thrombus, excluding patients with thrombus of other parts. Thus, there were more patients with ischemic cardiomyopathy and LV apical aneurysm in both groups. There was no significant difference of mitral E/E′ ratio, presence of LV apical aneurysm and LV SEC between the 2 groups. These factors were not associated with the early resolution of LV apical thrombi. In this present study, we demonstrated that the mobility of an LV apical thrombus was the most important parameter in predicting early thrombus resolution. Jugdutt et al.
16) reported the occurrence of embolic events associated with increased mobility of LV apical thrombi in patients with acute MI. Highly mobile thrombi may be associated with an increased risk of embolic events, and the thrombus subsequently becomes smaller in size secondary to the formation of emboli. A mobile thrombus may demonstrate a wider surface area than a non-mobile thrombus such that thrombolytic therapy can act better on. We observed that mural thrombi tended to have lesser mobility. Univariate analysis performed in our study showed that mural thrombi were associated with later resolution than round thrombi. The late resolution of LV apical thrombi was linked with an increased occurrence of adverse clinical events (HR, 5.727; 95% CI, 1.315–24.932; p=0.020). Persistent LV apical thrombi despite the administration of anticoagulants may indicate the presence of a favorable milieu for increased thrombogenicity. Notably, our study included 4 patients (5.2%) who developed symptomatic stroke during the administration of anticoagulants and 3 of these showed late thrombus resolution. Because mural thrombi might be more developed in irreversible cardiac condition, more heart failure admission and death might have occurred in this group.
This study has several limitations that mainly come from the nature of the study, in that it is a retrospective study involving a review of stored echocardiographic images and medical records. The echocardiographic examinations were performed at the discretion of the attending physician; thus, follow-up echocardiographic examinations after 1 month could not be performed at regular intervals and were performed intermittently. Additionally, the effectiveness of anticoagulant therapy evaluated by level of activated partial thromboplastin time or prothrombin time was not similar. Because the anticoagulant administered during the first month was relatively constant, we divided our study population into 2 groups based on the criterion of thrombus resolution following 1 month of therapy. The thrombus identification rate using echocardiographic studies might be lower than that using cardiac magnetic resonance imaging. However, transthoracic echocardiography is the most commonly used imaging modality that can be safely repeated. Thus, echocardiographic examination is feasible for the management and monitoring of LV apical thrombi. We propose that a prospective cohort study using a standardized anticoagulation protocol and regular follow-up imaging studies be done to accurately evaluate the time required for resolution of LV apical thrombi.
In conclusion, mobility of an LV apical thrombus was the most important parameter that served as a predictor of early thrombus resolution. Late resolution of LV apical thrombi was associated with poor long-term clinical outcomes.







 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download