Abstract
Background/Aims
The aim of this study was to determine the risk factors of multiple gastric polyps according to the histological classification of gastric polyps.
Methods
Subjects with multiple gastric polyps (at least three) during endoscopy were enrolled prospectively. They were assigned to a fundic gland polyp (FGP) group and hyperplastic polyp (HP) group based on a histological classification of gastric polyps. Helicobacter pylori (H. pylori) was confirmed by its histology. Serum gastrin was measured using the radioimmunoassay method. A questionnaire was taken regarding the intake of proton pump inhibitor and nonsteroidal anti-inflammatory drugs, alcohol, smoking history, and diet.
Results
Among the 60 subjects enrolled from 2015 to 2018 at Seoul National University Bungdang Hospital, 47 and 13 subjects were assigned to the FGP and HP groups, respectively. The H. pylori infection rate was 12.8% in the FGP group, which is lower than that in the HP group (69.2%, p<0.001). The gastrin level was higher in the HP group (194.7 pg/dL, range 50.6–387.8 pg/dL) than in the FGP group (57.4 pg/dL, range 24.8–79.0 pg/dL) (p=0.007). Histologically, neutrophil infiltration in the antrum and body of the stomach were higher in the HP group than in the FGP group (p=0.022 and p=0.030, respectively). In contrast, monocyte infiltration in the antrum and body of the stomach were higher in the FGP group than in the HP group (p=0.018 and p<0.001, respectively).
Most gastric polyps are usually asymptomatic. They are found incidentally in approximately 2% of upper endoscopies performed for unrelated reasons.1 A health check-up program designed to detect gastric cancer was implemented by the Korean government in 2001 for a biannual evaluation of Korean citizens aged more than 40 years. Since then, gastroscopy has become common and asymptomatic gastric polyps have been discovered increasingly. In recent years, the incidence of multiple gastric polyps has also increased.2 The most common type of gastric polyps detected by endoscopy are epithelial polyps, including fundic gland polyps (FGPs), hyperplastic polyp (HP), and gastric adenoma.3
FGPs account for 16–51% of gastric epithelial polyps, of which the endoscopic findings are usually multiple, small, transparent, sessile, and frequently located in the gastric fundus and body.45 A FGP is characterized histologically by microcysts and cystic dilatation of the glands lined with parietal and chief cells.
The pathophysiology of a FGP that usually occurs in normal, non-atrophic gastric mucosa678 is not known precisely.9 In sporadic FGPs, low-grade epithelial dysplasia has been described, but its prevalence is extremely rare (approximately 1%).10
HP represent 30–90% of gastric epithelial polyps. Their histologic features include elongation, twisting, branching, and cystic dilatation of the tortuous foveolae with inflamed stroma (so-called corkscrew appearance) lined by hyperplastic gastric mucin-containing epithelium.11 These polyps usually develop in patients with atrophic gastritis, Helicobacter pylori (H. pylori) associated gastritis, or gastric ulcer. They can also develop on the anastomotic site of Billroth I or II surgery because such sites are exposed continuously to bile reflux and a chronic inflammatory environment.45 HP can progress to cancer in 1.5–2.1% of cases. The risk of pre-cancerous lesions increases when the size is greater than 10 mm and pedunculated1213 in the peripheral mucosa of the polyp than in the polyp itself.314
Regarding the risk factors for the development of gastric polyps, a recent study reported that age and an unhealthy lifestyle of eating can affect the occurrence of gastric polyps.15 Nevertheless, comprehensive prospective studies on the risk factors (such as smoking, alcohol, proton pump inhibitor [PPI] and nonsteroidal anti-inflammatory drugs [NSAIDs] history, background gastritis status, gastrin levels together with gastrointestinal symptoms) of multiple gastric polyps have not been reported. This study hypothesized that the two categories of gastric polyps (FGP and HP) are quite different in terms of morphology, location in the stomach, pathology findings of gastric mucosa, and other risk factors. Therefore, the aim of this study was to determine the characteristics and risk factors (such as PPI and NSAIDs intake, H. pylori infection, serum gastrin concentration, alcohol, smoking, and diet) of multiple gastric polyps through a prospective study.
Subjects aged between 20 and 80 years old, who showed multiple gastric polyps by esophagogastroduodenoscopy from January 2015 to December 2018 at Seoul National University Bundang Hospital (diagnosed by NK), South Korea, were enrolled prospectively. Multiple gastric polyps were defined as the presence of three or more polyps on endoscopy. The status of atrophy and intestinal metaplasia of the background gastric mucosa was classified based on the Kimura-Takemoto classification (C-1, C-2, C-3, O-1, O-2, and O-3) for atrophy and Genta's classification (I and II) for intestinal metaplasia.
Subjects were excluded if their histological results revealed a diagnosis of gastritis or malignancy or if their serum gastrin level was not measured. Subjects with a prior history of peptic ulcer disease or benign gastric ulcer scars on endoscopy were excluded. Patients who took aspirin or clopidogrel until the endoscopy were also excluded. Only those patients who agreed to undergo a biopsy for H. pylori at the start of this study were enrolled. Fig. 1 presents a flow chart for selecting patients with multiple gastric polyps. Patients currently taking PPI were instructed to discontinue PPI for more than two weeks before undergoing endoscopy and biopsy. All subjects were Koreans. This study protocol was approved by the Ethics Committee of Seoul National University Bundang Hospital (IRB number: B-1712-438-103).
Subjects were classified into two groups (FGP group and HP group) if one or more gastric polyps were diagnosed as FGPs or HPs histologically (Fig. 2). If both FGP and HP appeared in the pathology results at the same time, the biopsy specimens were combined and the case was classified as one of the two that showed dominance.
For patients with the pathological findings of gastritis only, the pathologist (HSL) was requested to perform a pathology review to determine whether they were FGPs or HPs. FGPs were characterized histologically by one or more cystically dilated oxyntic glands composed of parietal and chief cells with a distorted glandular architecture and microcysts admixed with normal glands.9161718 HPs are characterized histologically by elongation, twisting, branching, and cystic dilatation of the foveolae with hyperplasia, with or without increased inflammatory cells in the lamina propria or surface erosions.1317
In addition, four biopsies were taken to determine the background gastric mucosal pathology and H. pylori infection (each specimen from the greater and lesser curvatures of the antrum and mid-body, respectively). They were fixed in formalin and assessed for the degree of inflammatory cells (neutrophil or monocyte) infiltration, atrophy, and intestinal metaplasia by H&E staining. The histological features of gastric mucosa were recorded using the updated Sydney system and classified as absent, mild, moderate, or marked (0–3, respectively).19
A current H. pylori infection was confirmed using the CLO test (Delta West, Bentley, Australia) pathology findings with four biopsy specimens on the body and the antrum (greater and lesser curvatures, respectively) by Giemsa staining. A history of eradication was taken by questionnaire. A prior infection was checked using a serum H. pylori IgG test. The patient was diagnosed with a past H. pylori infection if the H. pylori serology was positive but no bacteria were found by histology. The patient was defined as having a negative H. pylori infection with negative results of the CLO test or biopsy-based tests, negative H. pylori serology, and no history of H. pylori eradication.
All subjects responded to a questionnaire regarding their gastrointestinal symptoms, PPI and NSAIDs intake, alcohol consumption and smoking, and diet under the supervision of a well-trained interviewer soon after gastroscopy. In the case of PPI administration, the medicine and duration of intake were investigated. Symptoms (such as heartburn, acid regurgitation, chest pain, hoarseness, globus sensation, epigastric soreness, cough, dyspepsia, hematochezia, diarrhea, or no symptoms) at the time of the initial visit were chosen as the chief complaints. The dietary habits, such as salty and spicy food, were asked. Salty or spicy food was defined when the subjects added seasoning, such as salt or pepper.
The serum gastrin levels were measured using a radioimmunoassay method (Green Cross Medical Science Corp, Seoul, Korea). The normal values were 0–110 pg/mL.17 In this study, the serum gastrin levels were measured for most of the enrolled subjects except for two.
To analyze the correlations between the risk factors and each histologically classified multiple gastric polyp group, a chi-square test (Fisher's exact test) or Student's t-test, and a Mann-Whitney test were used for the categorical variables and continuous variables, respectively. Multiple logistic regression analysis was applied to reveal the relationship between the risk factors and each polyp group. p-values less than 0.05 were considered statistically significant. The OR with its 95% CI was used as a measure of an association. SPSS for windows version 19.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
A total of 72 subjects diagnosed with multiple gastric polyp by endoscopy were enrolled during the study period. Twelve subjects were excluded from this study. Therefore, the pathologic results of 60 patients with FGPs or HP were obtained through a gastric biopsy. Their basic characteristics are summarized in Table 1. Two (3.33%) subjects took NSAIDs: one subject in the FGP group and one in the HP group.
The endoscopic features of the two groups were compared; the results are shown in Table 2. The FGPs was distributed mainly in the body of the stomach (n=23, 48.9%) and both the body and fundus (n=16, 34.0%), whereas HPs were distributed mainly in the body of the stomach (n=5, 38.5%) and in both the body and antrum (n=4, 30.8%), showing a significant difference between the two groups (p=0.005). On the other hand, there were no significant differences in the number receiving endoscopy, number of polyps, number of subjects with increased number of polyps during the study, or endoscopic classification of atrophy and intestinal metaplasia between the two groups.
In the endoscopic classification of atrophic gastritis (AG) and intestinal metaplasia (IM), one (2.1%) subject was AG C-1 in the FGP and one (7.7%) subject was C-3 in the HP. There was no IM in either group.
The risk factors of FGPs and HPs were compared; the results are summarized in Table 1 and Fig 3. The proportion of H. pylori infection was significantly lower in the FGP (n=6, 12.8%) than in the HP group (n=9, 69.2%) (p<0.001). The median serum gastrin level (pg/dL) was significantly higher in the HP group (194.7, range 50.6–387.8) than in the FGP (57.4, range 24.8–79.0) (p=0.007). Histologically, neutrophil infiltration in the antrum (n=3, 23.1%) (p=0.022) and body of the stomach (n=4, 30.8%) (p=0.030) were significantly higher in the HP group than those of the FGP group (both n=1, 2.1%). In addition, the HP group showed higher intestinal metaplasia in the antrum (n=3, 23.1%) (p=0.010) and in the body of the stomach (n=2, 5.4%) (p=0.024) than the FGP group (both n=0, 0.0%). On the other hand, monocyte infiltration in the antrum (n=36, 76.6%) (p=0.018) and body of the stomach (n=35, 75.5%) (p<0.001) in the FGP group were significantly higher than those in the HP group (both n=9, 69.2%). On the other hand, there was no statistical difference in age, sex, PPI and NSAIDs intake, smoking, alcohol, or diet habits observed between the two groups.
Gastrin is associated with a H. pylori infection. A H. pylori infection causes HPs and oxyntic mucosal atrophy.20 Oxyntic mucosal atrophy then leads to hypergastrinemia due to a decrease in gastric acidity.21 Gastrin also has a tropic effect on enterochromaffin-like cells and oxyntic mucosa, and has been reported to play a pro-carcinogenic role in preclinical studies.2122 Based on the pathology that serum gastrin acts as a tropic factor, HPs usually occur in chronic gastritis associated with a H. pylori infection. The development of HPs is a process of chronic inflammation and re-epithelization associated with a H. pylori infection and hypergastrinemia.
In this study, the HP group had a more frequent H. pylori infection and higher serum gastrin concentration than the FGP group. In addition, the mean serum gastrin concentration in the H. pylori infection positive group (mean, 102.3 pg/dL; range, 26.8–363.4 pg/dL) was significantly higher than that in the H. pylori infection negative group (mean, 56.1 pg/dL; range, 2.5–79.1 pg/dL) (p=0.010). On the other hand, the rate of H. pylori infection in each group was lower than the Korean average of 54.4% in 2001. This might be related to the expansion of H. pylori eradication treatments and the socioeconomic status of the subjects.
Previous studies have suggested that the use of PPI is associated with FGPs.2324 In addition, the incidence of FGP has increased with increasing duration of PPI administration.23252627 The mechanism involved in the increase in the incidence of FGP by PPI has not been elucidated in detail. The increase in gastrin production secondary to acid suppression can cause an enlargement of enterochromaffin-like cells and parietal cells and decrease the number of chief cells without affecting the A-like cells.2829 In addition, secretory products and parietal cell protrusions with the development of gland dilatation and cystic changes as alterations might precede a FGP.30 In this study, no subject in the FGP group with a PPI intake had parietal cell hyperplasia histologically. In subjects taking PPI, the serum gastrin concentration was significantly higher than that in subjects not taking PPI (78.9 pg/mL [95% CI, 44.1–177.3 pg/mL] vs. 55.0 pg/mL [95% CI, 44.2–77.5 pg/mL], p=0.048) (Table 3). On the other hand, there was no significant difference in the serum gastrin level during PPI administration between the FGP group and HP group in this study. The reason for this was that a large number of subjects in the HP group were symptomatic and taking PPI at the beginning of the study: six (75.0%) out of eight subjects who took PPI in the HP group had a history of less than six months of PPI intake.
In the HP group, the number of patients with symptoms, such as heartburn and acid regurgitation, was significantly higher than that in the FGP at the first visit. Regarding the relationship between the PPI intake and initial symptoms, 28 (46.7%) subjects who took PPIs answered that they had abdominal symptoms with a significant difference compared to the 32 (53.3%) subjects who did not take PPI (p=0.024).
In 2001, Abraham et al.31 performed a histological evaluation of the surrounding mucosa in patients with HPs and reported the presence of IM and gastritis in 37% and 85% of subjects, respectively. Dirschmid et al.32 in 2006 reported autoimmune gastritis in 51.3% of corpus mucosa patients and chronic active H. pylori gastritis in 37.3% of patients with HPs.
In this study, IMs on the antrum and body were significantly higher in the HP group (n=3, 23.1% and n=2, 15.4%, respectively) than in the FGP group (n=0, 0.0%, both antrum and body) (p=0.001 and p=0.024, respectively). AGs on the antrum and body were 23.1% and 15.4%, respectively, in the HP group, showing no significant difference from those in the FGP group. This study also showed that the infiltration of neutrophils on the antrum (n=3, 23.1%) (p=0.022) and body of the stomach (n=3, 23.1%) (p=0.03) in subjects with HPs were significantly higher than those with FGPs. In addition, HPs were distributed mainly in the body (n=5, 38.5%) and both body antrum (n=6, 46.2%).
On the other hand, a gastric mucosal biopsy of the antrum and body around the polyp in the FGP group showed only the infiltration of mild monocytes with little infiltration of neutrophils. Less IM was observed compared to that in the HP group. Therefore, it was histologically confirmed that a FGP occurs in the background of the normal gastric mucosa.
In this study, neither age nor sex was associated with FGPs. On the other hand, somatic mutations and alleles of adenomatous polyposis coli gene in the FGP of familial adenomatous polyposis patients have been reported since 1990.3334 Adenomatous polyposis coli gene alterations are less common in sporadic cases than in familial adenomatous polyposis-associated cases, in which a β-catenin gene mutation is frequent.3536 Abraham et al.36 identified mutations in exon 3 of the β-catenin gene in 52 (91%) out of 57 sporadic FGPs of the stomach. On the other hand, it is unclear if the occurrence of FGP is due to the intrinsic mutation of the β-catenin gene or exposure to other carcinogenic stimuli.
In addition, the fact that multiple gastric polyps are found in peri-menstrual women suggests that changes in the sex hormones might be associated with the development of gastric polyps. Previous studies on colon polyps and estrogen have hypothesized that estrogen might have a direct effect on the growth of colon cancer cells in the colonic mucosa.3738 Recently, Woodson et al.39 reported that hormone replacement therapy can reduce the risk of recurrence of colon polyps in older women. On the other hand, the effects of sex hormones on gastric polyps have not been studied in detail yet; further studies will be needed.
Table 4 lists the characteristics of studies regarding the association between gastric polyps and their risk factors. Among previous studies1332 of multiple gastric polyps, retrospective studies of FGPs or HPs were the most common. Detailed PPI intake history, gastrin level, and the degree of gastric mucosal inflammation by a biopsy were not evaluated clearly. Therefore, this study had the following strengths. First, the subjects were enrolled by one endoscopic expert (NK) in a single institute. Second, the risk factors were collected prospectively with a questionnaire by a clinical research assistant. Third, the classification was performed for the antrum and body. Fourth, serum gastrin and histologic analyses were performed for all subjects with multiple gastric polyps. This study is believed to be the first prospective study in Korea to analyze the risk factors of multiple gastric polyps with a histological review, serum gastrin, H. pylori infection, PPI history, and other risk factors together.
The limitation of this study was that it was difficult to identify accurately the period of taking PPI prior to study enrollment because PPI administration was investigated through a questionnaire. Second, the number of subjects enrolled in this study was small, which limited the statistical analysis of the risk factors, particularly in the HP group. In addition, the precise cause and clinical significance of mild infiltration of monocytes in the surrounding gastric mucosa of the FGP remain unclear. The immune mechanism and external stimuli of the gastrointestinal tract might play a role.
In conclusion, this prospective study suggests that HPs arise due to inflammation caused by H. pylori and that FGPs are not related to PPI intake or epidemiologic causes. The association between FGPs and monocyte infiltration requires further study with a larger number of cases.
Figures and Tables
 | Fig. 1Flow chart showing the selection of patients with multiple gastric polyps classified by histopathology. |
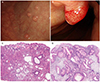 | Fig. 2Endoscopy findings of a representative fundic gland polyp (FGP) (A) or hyperplastic polyp (HP) (B). (A) Endoscopic view of a FGP showing more than 10 polyps of 1 cm or less throughout the body and antrum. (B) Endoscopic view of a HP showing hyperemic polypoid lesions, approximately 1 cm in size, on the body. Histology findings of representative FGP (C) or HP (D). (C) Representative photomicrograph of a FGP showing the proliferation of oxyntic glands with a cystic dilation and disordered architecture (H&E, ×40). (D) Representative photomicrograph of a HP showing dilated and hyperplastic foveolar glands with edematous and inflamed stroma (H&E, ×40). |
 | Fig. 3Multivariate analysis between the fundic gland polyp group and hyperplastic polyp group. H. pylori, Helicobacter pylori; AG, atrophic gastritis; IM, intestinal metaplasia. aMeans statistical significance. |
ACKNOWLEDGEMENT
We are grateful to Ji Young Kim and So Dam Sohn for assistance with data collecting.
Notes
References
1. Dekker W. Clinical relevance of gastric and duodenal polyps. Scand J Gastroenterol Suppl. 1990; 178:7–12.

3. Goddard AF, Badreldin R, Pritchard DM, Walker MM, Warren B, British Society. The management of gastric polyps. Gut. 2010; 59:1270–1276.

4. Borch K, Skarsgård J, Franzén L, Mårdh S, Rehfeld JF. Benign gastric polyps: morphological and functional origin. Dig Dis Sci. 2003; 48:1292–1297.
5. Stolte M, Sticht T, Eidt S, Ebert D, Finkenzeller G. Frequency, location, and age and sex distribution of various types of gastric polyp. Endoscopy. 1994; 26:659–665.

6. Sipponen P, Laxén F, Seppälä K. Cystic ‘hamartomatous’ gastric polyps: a disorder of oxyntic glands. Histopathology. 1983; 7:729–737.

7. Lee RG, Burt RW. The histopathology of fundic gland polyps of the stomach. Am J Clin Pathol. 1986; 86:498–503.

8. Haruma K, Sumii K, Yoshihara M, Watanabe C, Kajiyama G. Gastric mucosa in female patients with fundic glandular polyps. J clin gastroenterol. 1991; 13(5):565–569.

9. Odze RD, Marcial MA, Antonioli D. Gastric fundic gland polyps: a morphological study including mucin histochemistry, stereometry, and MIB-1 immunohistochemistry. Hum Pathol. 1996; 27:896–903.

10. Wu TT, Kornacki S, Rashid A, Yardley JH, Hamilton SR. Dysplasia and dysregulation of proliferation in foveolar and surface epithelia of fundic gland polyps from patients with familial adenomatous polyposis. Am J Surg Pathol. 1998; 22:293–298.

12. Han AR, Sung CO, Kim KM, et al. The clinicopathological features of gastric hyperplastic polyps with neoplastic transformations: a suggestion of indication for endoscopic polypectomy. Gut Liver. 2009; 3:271–275.

13. Stolte M. Hyperplastic polyps of the stomach: associations with histologic patterns of gastritis and gastric atrophy. Am J Surg Pathol. 2001; 25:1342–1344.

14. Carmack SW, Genta RM, Graham DY, Lauwers GY. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol. 2009; 6:331–341.

15. Cao W, Hou G, Zhang X, San H, Zheng J. Potential risk factors related to the development of gastric polyps. Immunopharmacol Immunotoxicol. 2018; 40:338–343.

16. Park DY, Lauwers GY. Gastric polyps: classification and management. Arch Pathol Lab Med. 2008; 132:633–640.

17. Hongo M, Fujimoto K, Gastric Polyps. Incidence and risk factor of fundic gland polyp and hyperplastic polyp in long-term proton pump inhibitor therapy: a prospective study in Japan. J Gastroenterol. 2010; 45:618–624.

19. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney system. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol. 1996; 20:1161–1181.
20. Korman MG, Strickland RG, Hansky J. Serum gastrin in chronic gastritis. Br Med J. 1971; 2:16–18.

21. Kim BC, Jung SW, Kim JB, et al. Serum gastrin levels in different stages of distal gastric carcinogenesis: is there a role for serum gastrin in tumor growth? Turk J Gastroenterol. 2014; 25:611–618.

22. Watson SA, Grabowska AM, El-Zaatari M, Takhar A. Gastrin - active participant or bystander in gastric carcinogenesis? Nat Rev Cancer. 2006; 6:936–946.

23. Jalving M, Koornstra JJ, Wesseling J, Boezen HM, DE Jong S, Kleibeuker JH. Increased risk of fundic gland polyps during long-term proton pump inhibitor therapy. Aliment Pharmacol Ther. 2006; 24:1341–1348.

24. el-Zimaity HM, Jackson FW, Graham DY. Fundic gland polyps developing during omeprazole therapy. Am J Gastroenterol. 1997; 92:1858–1860.
25. Tran-Duy A, Spaetgens B, Hoes AW, de Wit NJ, Stehouwer CD. Use of proton pump inhibitors and risks of fundic gland polyps and gastric cancer: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016; 14:1706–1719.e5.

26. Zelter A, Fernández JL, Bilder C, et al. Fundic gland polyps and association with proton pump inhibitor intake: a prospective study in 1,780 endoscopies. Dig Dis Sci. 2011; 56:1743–1748.

27. Martin FC, Chenevix-Trench G, Yeomans ND. Systematic review with meta-analysis: fundic gland polyps and proton pump inhibitors. Aliment Pharmacol Ther. 2016; 44:915–925.

28. Masaoka T, Suzuki H, Hibi T. Gastric epithelial cell modality and proton pump inhibitor. J Clin Biochem Nutr. 2008; 42:191–196.

29. Ally MR, Veerappan GR, Maydonovitch CL, et al. Chronic proton pump inhibitor therapy associated with increased development of fundic gland polyps. Dig Dis Sci. 2009; 54:2617–2622.

30. Cats A, Schenk BE, Bloemena E, et al. Parietal cell protrusions and fundic gland cysts during omeprazole maintenance treatment. Hum Pathol. 2000; 31:684–690.

31. Abraham SC, Singh VK, Yardley JH, Wu TT. Hyperplastic polyps of the stomach: associations with histologic patterns of gastritis and gastric atrophy. Am J Surg Pathol. 2001; 25:500–507.

32. Dirschmid K, Platz-Baudin C, Stolte M. Why is the hyperplastic polyp a marker for the precancerous condition of the gastric mucosa? Virchows Arch. 2006; 448:80–84.

33. Abraham SC, Nobukawa B, Giardiello FM, Hamilton SR, Wu TT. Fundic gland polyps in familial adenomatous polyposis: neoplasms with frequent somatic adenomatous polyposis coli gene alterations. Am J Pathol. 2000; 157:747–754.
34. Toyooka M, Konishi M, Kikuchi-Yanoshita R, Iwama T, Miyaki M. Somatic mutations of the adenomatous polyposis coli gene in gastroduodenal tumors from patients with familial adenomatous polyposis. Cancer Res. 1995; 55:3165–3170.
35. Koch A, Denkhaus D, Albrecht S, Leuschner I, von Schweinitz D, Pietsch T. Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the beta-catenin gene. Cancer Res. 1999; 59:269–273.
36. Abraham SC, Nobukawa B, Giardiello FM, Hamilton SR, Wu TT. Sporadic fundic gland polyps: common gastric polyps arising through activating mutations in the beta-catenin gene. Am J Pathol. 2001; 158:1005–1010.
37. Foley EF, Jazaeri AA, Shupnik MA, Jazaeri O, Rice LW. Selective loss of estrogen receptor beta in malignant human colon. Cancer Res. 2000; 60:245–248.
38. Singh S, Paraskeva C, Gallimore PH, Sheppard MC, Langman MJ. Differential growth response to oestrogen of premalignant and malignant colonic cell lines. Anticancer Res. 1994; 14:1037–1041.
39. Woodson K, Lanza E, Tangrea JA, et al. Hormone replacement therapy and colorectal adenoma recurrence among women in the polyp prevention trial. J Natl Cancer Inst. 2001; 93:1799–1805.

40. Graham JR. Gastric polyposis: onset during long-term therapy with omeprazole. Med J Aust. 1992; 157:287–288.

41. Choudhry U, Boyce HW Jr, Coppola D. Proton pump inhibitor-associated gastric polyps: a retrospective analysis of their frequency, and endoscopic, histologic, and ultrastructural characteristics. Am J Clin Pathol. 1998; 110:615–621.

42. Vieth M, Stolte M. Fundic gland polyps are not induced by proton pump inhibitor therapy. Am J Clin Pathol. 2001; 116(5):716–720.

43. Peura DA, Metz DC, Dabholkar AH, Paris MM, Yu P, Atkinson SN. Safety profile of dexlansoprazole MR, a proton pump inhibitor with a novel dual delayed release formulation: global clinical trial experience. Aliment Pharmacol Ther. 2009; 30(10):1010–1021.

44. Hsu WH, Wu IC, Kuo CH, et al. Influence of proton pump inhibitor use in gastrointestinal polyps. Kaohsiung J Med Sci. 2010; 26:76–83.

45. Cao H, Qu R, Zhang Z, et al. Sporadic fundic gland polyps are not associated with proton pump inhibitors therapy but negatively correlate with Helicobacter pylori infection in China. Chin Med J. 2014; 127:1239–1243.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download