1. Dauwe PB, Pulikkottil BJ, Lavery L, Stuzin JM, Rohrich RJ. Does hyperbaric oxygen therapy work in facilitating acute wound healing: a systematic review. Plast Reconstr Surg. 2014; 133:208e–215e.
2. Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy. N Engl J Med. 1996; 334:1642–1648.

3. Howard MA, Asmis R, Evans KK, Mustoe TA. Oxygen and wound care: a review of current therapeutic modalities and future direction. Wound Repair Regen. 2013; 21:503–511.

4. Hopf HW, Gibson JJ, Angeles AP, Constant JS, Feng JJ, Rollins MD, et al. Hyperoxia and angiogenesis. Wound Repair Regen. 2005; 13:558–564.

5. Jan AM, Sándor GK, Iera D, Mhawi A, Peel S, Evans AW, et al. Hyperbaric oxygen results in an increase in rabbit calvarial critical sized defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 101:144–149.

6. Jan A, Sándor GK, Brkovic BB, Peel S, Evans AW, Clokie CM. Effect of hyperbaric oxygen on grafted and nongrafted calvarial critical-sized defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107:157–163.

7. Pedersen TO, Xing Z, Finne-Wistrand A, Hellem S, Mustafa K. Hyperbaric oxygen stimulates vascularization and bone formation in rat calvarial defects. Int J Oral Maxillofac Surg. 2013; 42:907–914.

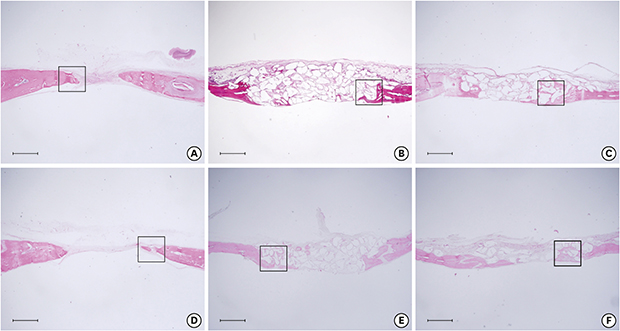
8. Chang H, Oh SE, Oh S, Hu KS, Kim S. Four-week histologic evaluation of grafted calvarial defects with adjunctive hyperbaric oxygen therapy in rats. J Periodontal Implant Sci. 2016; 46:244–253.

9. Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert Rev Med Devices. 2006; 3:49–57.

10. Palti A, Hoch T. A concept for the treatment of various dental bone defects. Implant Dent. 2002; 11:73–78.

11. Kim S, Jung UW, Lee YK, Choi SH. Effects of biphasic calcium phosphate bone substitute on circumferential bone defects around dental implants in dogs. Int J Oral Maxillofac Implants. 2011; 26:265–273.
12. Choi H, Park NJ, Jamiyandorj O, Choi KH, Hong MH, Oh S, et al. Improvement of osteogenic potential of biphasic calcium phosphate bone substitute coated with two concentrations of expressed recombinant human bone morphogenetic protein 2. J Periodontal Implant Sci. 2012; 42:119–126.

13. Shin YS, Seo JY, Oh SH, Kim JH, Kim ST, Park YB, et al. The effects of ErhBMP-2-/EGCG-coated BCP bone substitute on dehiscence around dental implants in dogs. Oral Dis. 2014; 20:281–287.

14. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008; 8:1011–1018.

15. Kaneko H, Arakawa T, Mano H, Kaneda T, Ogasawara A, Nakagawa M, et al. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone. 2000; 27:479–486.

16. Smucker JD, Rhee JM, Singh K, Yoon ST, Heller JG. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine. 2006; 31:2813–2819.

17. Jin P, Wu H, Xu G, Zheng L, Zhao J. Epigallocatechin-3-gallate (EGCG) as a pro-osteogenic agent to enhance osteogenic differentiation of mesenchymal stem cells from human bone marrow: an
in vitro study. Cell Tissue Res. 2014; 356:381–390.

18. Oka Y, Iwai S, Amano H, Irie Y, Yatomi K, Ryu K, et al. Tea polyphenols inhibit rat osteoclast formation and differentiation. J Pharmacol Sci. 2012; 118:55–64.

19. Xiao SJ, Textor M, Spencer ND, Sigrist H. Covalent attachment of cell-adhesive, (Arg-Gly-Asp)-containing peptides to titanium surfaces. Langmuir. 1998; 14:5507–5516.

20. Durrieu MC, Pallu S, Guillemot F, Bareille R, Amédée J, Baquey CH, et al. Grafting RGD containing peptides onto hydroxyapatite to promote osteoblastic cells adhesion. J Mater Sci Mater Med. 2004; 15:779–786.

21. Jan A, Sándor GK, Brkovic BB, Peel S, Kim YD, Xiao WZ, et al. Effect of hyperbaric oxygen on demineralized bone matrix and biphasic calcium phosphate bone substitutes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:59–66.

22. Sirin Y, Olgac V, Dogru-Abbasoglu S, Tapul L, Aktas S, Soley S. The influence of hyperbaric oxygen treatment on the healing of experimental defects filled with different bone graft substitutes. Int J Med Sci. 2011; 8:114–125.

23. Develıoğlu H, Saraydin SU, Bolayir G, Dupoirieux L. Assessment of the effect of a biphasic ceramic on bone response in a rat calvarial defect model. J Biomed Mater Res A. 2006; 77A:627–631.

24. Sawai T, Niimi A, Takahashi H, Ueda M. Histologic study of the effect of hyperbaric oxygen therapy on autogenous free bone grafts. J Oral Maxillofac Surg. 1996; 54:975–981.

25. Fok TC, Jan A, Peel SA, Evans AW, Clokie CM, Sándor GK. Hyperbaric oxygen results in increased vascular endothelial growth factor (VEGF) protein expression in rabbit calvarial critical-sized defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 105:417–422.

26. Gosain AK, Song L, Yu P, Mehrara BJ, Maeda CY, Gold LI, et al. Osteogenesis in cranial defects: reassessment of the concept of critical size and the expression of TGF-beta isoforms. Plast Reconstr Surg. 2000; 106:360–371.

27. Cooper GM, Mooney MP, Gosain AK, Campbell PG, Losee JE, Huard J. Testing the critical size in calvarial bone defects: revisiting the concept of a critical-size defect. Plast Reconstr Surg. 2010; 125:1685–1692.

28. Dånmark S, Finne-Wistrand A, Wendel M, Arvidson K, Albertsson AC, Mustafa K. Osteogenic differentiation by rat bone marrow stromal cells on customized biodegradable polymer scaffolds. J Bioact Compat Polym. 2010; 25:207–223.

29. Mardas N, Kostopoulos L, Karring T. Bone and suture regeneration in calvarial defects by e-PTFE-membranes and demineralized bone matrix and the impact on calvarial growth: an experimental study in the rat. J Craniofac Surg. 2002; 13:453–462.

30. Humber CC, Sándor GK, Davis JM, Peel SA, Brkovic BM, Kim YD, et al. Bone healing with an in situ-formed bioresorbable polyethylene glycol hydrogel membrane in rabbit calvarial defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:372–384.








 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download