1. Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001; 24(1):1121–1159.

2. Millan MJ. The epigenetic dimension of Alzheimer's disease: causal, consequence, or curiosity? Dialogues Clin Neurosci. 2014; 16(3):373–393.

3. Zhang K, Schrag M, Crofton A, Trivedi R, Vinters H, Kirsch W. Targeted proteomics for quantification of histone acetylation in Alzheimer's disease. Proteomics. 2012; 12(8):1261–1268.

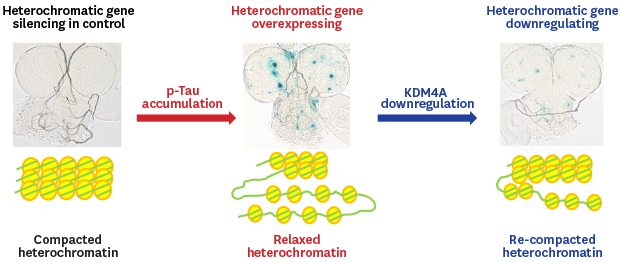
4. Chanu SI, Sarkar S. Targeted downregulation of dMyc suppresses pathogenesis of human neuronal tauopathies in drosophila by limiting heterochromatin relaxation and tau hyperphosphorylation. Mol Neurobiol. 2017; 54(4):2706–2719.

5. Frost B, Hemberg M, Lewis J, Feany MB. Tau promotes neurodegeneration through global chromatin relaxation. Nat Neurosci. 2014; 17(3):357–366.

6. Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A. 2001; 98(22):12596–12601.

7. Narayan P, Dragunow M. Alzheimer's disease and histone code alterations. Adv Exp Med Biol. 2017; 978:321–336.

8. Millan MJ. An epigenetic framework for neurodevelopmental disorders: from pathogenesis to potential therapy. Neuropharmacology. 2013; 68:2–82.

9. Gräff J, Rei D, Guan JS, Wang WY, Seo J, Hennig KM, et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012; 483(7388):222–226.

10. Day JJ, Sweatt JD. Epigenetic mechanisms in cognition. Neuron. 2011; 70(5):813–829.

11. Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007; 447(7141):178–182.

12. Johnson AA, Sarthi J, Pirooznia SK, Reube W, Elefant F. Increasing Tip60 HAT levels rescues axonal transport defects and associated behavioral phenotypes in a Drosophila Alzheimer's disease model. J Neurosci. 2013; 33(17):7535–7547.

13. Xu S, Wilf R, Menon T, Panikker P, Sarthi J, Elefant F. Epigenetic control of learning and memory in Drosophila by Tip60 HAT action. Genetics. 2014; 198(4):1571–1586.

14. Xu S, Elefant F. Tip off the HAT-epigenetic control of learning and memory by Drosophila Tip60. Fly (Austin). 2015; 9(1):22–28.
15. Xiong Y, Zhao K, Wu J, Xu Z, Jin S, Zhang YQ. HDAC6 mutations rescue human tau-induced microtubule defects in Drosophila. Proc Natl Acad Sci U S A. 2013; 110(12):4604–4609.

16. Jarome TJ, Lubin FD. Histone lysine methylation: critical regulator of memory and behavior. Rev Neurosci. 2013; 24(4):375–387.

17. Mastroeni D, Delvaux E, Nolz J, Tan Y, Grover A, Oddo S, et al. Aberrant intracellular localization of H3k4me3 demonstrates an early epigenetic phenomenon in Alzheimer's disease. Neurobiol Aging. 2015; 36(12):3121–3129.
18. Park SY, Park JW, Chun YS. Jumonji histone demethylases as emerging therapeutic targets. Pharmacol Res. 2016; 105:146–151.

19. Zheng Y, Hsu FN, Xu W, Xie XJ, Ren X, Gao X, et al. A developmental genetic analysis of the lysine demethylase KDM2 mutations in Drosophila melanogaster. Mech Dev. 2014; 133:36–53.

20. Herz HM, Morgan M, Gao X, Jackson J, Rickels R, Swanson SK, et al. Histone H3 lysine-to-methionine mutants as a paradigm to study chromatin signaling. Science. 2014; 345(6200):1065–1070.

21. Lloret-Llinares M, Carré C, Vaquero A, de Olano N, Azorín F. Characterization of Drosophila melanogaster JmjC+N histone demethylases. Nucleic Acids Res. 2008; 36(9):2852–2863.

22. Lin CH, Li B, Swanson S, Zhang Y, Florens L, Washburn MP, et al. Heterochromatin protein 1a stimulates histone H3 lysine 36 demethylation by the Drosophila KDM4A demethylase. Mol Cell. 2008; 32(5):696–706.

23. Song Z, Guan B, Bergman A, Nicholson DW, Thornberry NA, Peterson EP, et al. Biochemical and genetic interactions between Drosophila caspases and the proapoptotic genes rpr, hid, and grim. Mol Cell Biol. 2000; 20(8):2907–2914.
24. Nahm M, Kim S, Paik SK, Lee M, Lee S, Lee ZH, et al. dCIP4 (Drosophila Cdc42-interacting protein 4) restrains synaptic growth by inhibiting the secretion of the retrograde Glass bottom boat signal. J Neurosci. 2010; 30(24):8138–8150.

25. Blackman RK, Sanicola M, Raftery LA, Gillevet T, Gelbart WM. An extensive 3′ cis-regulatory region directs the imaginal disk expression of decapentaplegic, a member of the TGF-beta family in Drosophila. Development. 1991; 111(3):657–666.

26. Chatterjee S, Sang TK, Lawless GM, Jackson GR. Dissociation of tau toxicity and phosphorylation: role of GSK-3beta, MARK and Cdk5 in a Drosophila model. Hum Mol Genet. 2009; 18(1):164–177.
27. Sang TK, Jackson GR. Drosophila models of neurodegenerative disease. NeuroRx. 2005; 2(3):438–446.

28. Chan HY, Bonini NM. Drosophila models of human neurodegenerative disease. Cell Death Differ. 2000; 7(11):1075–1080.

29. Crowther DC, Kinghorn KJ, Page R, Lomas DA. Therapeutic targets from a Drosophila model of Alzheimer's disease. Curr Opin Pharmacol. 2004; 4(5):513–516.

30. Scherzer-Attali R, Pellarin R, Convertino M, Frydman-Marom A, Egoz-Matia N, Peled S, et al. Complete phenotypic recovery of an Alzheimer's disease model by a quinone-tryptophan hybrid aggregation inhibitor. PLoS One. 2010; 5(6):e11101.

31. Crowther DC, Kinghorn KJ, Miranda E, Page R, Curry JA, Duthie FA, et al. Intraneuronal Abeta, non-amyloid aggregates and neurodegeneration in a Drosophila model of Alzheimer's disease. Neuroscience. 2005; 132(1):123–135.
32. Frydman-Marom A, Levin A, Farfara D, Benromano T, Scherzer-Attali R, Peled S, et al. Orally administrated cinnamon extract reduces β-amyloid oligomerization and corrects cognitive impairment in Alzheimer's disease animal models. PLoS One. 2011; 6(1):e16564.

33. Lu BY, Ma J, Eissenberg JC. Developmental regulation of heterochromatin-mediated gene silencing in Drosophila. Development. 1998; 125(12):2223–2234.

34. Lin CH, Paulson A, Abmayr SM, Workman JL. HP1a targets the Drosophila KDM4A demethylase to a subset of heterochromatic genes to regulate H3K36me3 levels. PLoS One. 2012; 7(6):e39758.

35. Colmenares SU, Swenson JM, Langley SA, Kennedy C, Costes SV, Karpen GH. Drosophila histone demethylase KDM4A has enzymatic and non-enzymatic roles in controlling heterochromatin integrity. Dev Cell. 2017; 42(2):156–169.e5.

36. Lorbeck MT, Singh N, Zervos A, Dhatta M, Lapchenko M, Yang C, et al. The histone demethylase Dmel\Kdm4A controls genes required for life span and male-specific sex determination in Drosophila. Gene. 2010; 450(1-2):8–17.

37. Fujiwara K, Fujita Y, Kasai A, Onaka Y, Hashimoto H, Okada H, et al. Deletion of JMJD2B in neurons leads to defective spine maturation, hyperactive behavior and memory deficits in mouse. Transl Psychiatry. 2016; 6(3):e766.

38. Zheng Y, Xue Y, Ren X, Liu M, Li X, Jia Y, et al. The lysine demethylase dKDM2 is non-essential for viability, but regulates circadian rhythms in
Drosophila
. Front Genet. 2018; 9:354.








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download