This article has been
cited by other articles in ScienceCentral.
Abstract
Postinfectious glomerulonephritis (PIGN) is most commonly caused by Streptococcus pyogenes in children, but PIGN associated with other pathogens has been described in the literature. A previously healthy 6-year-old boy was admitted with complaints of cough, fever, and right chest pain. The patient was diagnosed with pneumococcal bacteremia and influenza A virus infection and treated with antibiotics and antiviral agent. During hospitalization, generalized edema, hematuria, proteinuria, and increased blood pressure were observed; therefore, we started administering diuretics. The boy was discharged with gross hematuria, and even microscopic hematuria disappeared 14 weeks after discharge. We report a case of PIGN associated with bacteremic pneumococcal pneumonia and influenza A virus infection in children. A urine test and blood pressure measurement should be considered for the early detection of PIGN in children with pneumococcal or influenza A virus infection when they present with nephritic symptoms.
초록
감염 후 사구체신염(postinfectious glomerulonephritis, PIGN)은 주로 Streptococcus pyogenes에 의해 발생하지만, 다른 병원체와 관련된 PIGN도 문헌에서 확인된다. 기침, 발열, 우측 흉통을 주소로 내원한 6세 남아에서 폐구균 및 인플루엔자 A 바이러스의 감염이 확인되었고 항생제와 항바이러스제가 투약되었다. 입원 중 전신 부종, 혈뇨, 단백뇨, 혈압의 상승이 관찰되었고, 따라서 이뇨제가 투약되었다. 환자는 육안적 혈뇨가 관찰되는 상태로 퇴원하였고, 퇴원 후 14주에 현미경적 혈뇨까지 사라졌다. 폐구균 또는 인플루엔자 A 바이러스 감염이 확인된 환아에서 신염의 증상이 보이는 경우 감염 후 사구체신염의 조기발견을 위해 소변검사 및 혈압의 측정이 이루어져야 한다. 본 저자들은 폐구균 및 인플루엔자 A 바이러스에 의한 감염 후 사구체신염 사례를 경험하였기에 이를 보고하는 바이다.
Keywords: Streptococcus pneumoniae, Glomerulonephritis, Influenza, Pneumonia, Children
INTRODUCTION
Postinfectious glomerulonephritis (PIGN) is the most common form of acute glomerulonephritis (AGN) in children. PIGN is most commonly caused by
Streptococcus pyogenes and is often referred to as poststreptococcal glomerulonephritis (PSGN). The classic clinical manifestation of PSGN is the appearance of hematuria, proteinuria, hypertension, edema, and renal dysfunction after 1–2 weeks of throat infection or 2–6 weeks after skin infection by
S. pyogenes.
1) PIGN caused by other pathogens, including
Streptococcus pneumoniae, has been reported occasionally.
23456789) In this study, we report a case of PIGN associated with
S. pneumoniae and influenza A virus infection in a 6-year-old Korean child and review the cases reported to date.
CASE
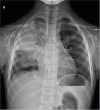
A 6-year-old boy came to the emergency room after 2 weeks of cough and 3 days of high fever. Right chest pain, which was more severe upon holding his breath, began soon after the onset of fever. His vital signs were as follows: 141/73 mmHg blood pressure (BP), 155 beats/minute pulse rate, 28 breaths/minute respiration rate, and 39.3°C body temperature. Upon physical examination, decreased breath sounds with mild crackle were auscultated on the right upper lung field. The initial chest X-ray showed lobar consolidation on the right upper lobe and a small amount of pleural effusion (
Fig. 1). In the peripheral blood analyses, the number of leukocytes was 16,250/mm
3 (83.5% neutrophils, 11.7% lymphocytes, and 4.6% monocytes), and the level of C-reactive protein was 20.04 mg/dL. The levels of blood urea nitrogen and creatinine were 13.0 and 0.54 mg/dL, respectively. The serum levels of total protein and albumin were 6.0 and 2.7 mg/dL, respectively. Urinalysis showed albumin 2+, occult blood 2+, and leukocyte 1+. In the sediment urine test, 10–19 red blood cells (RBCs) and 5–9 white blood cells (WBCs) per high power field (HPF) were observed. An influenza virus rapid antigen test of a nasopharyngeal swab sample was positive for influenza A virus. We started oseltamivir (45 mg, twice a day) to treat the influenza infection and empirical antibiotics including ampicillin/sulbactam (1,200 mg, four times a day) and roxithromycin (50 mg, twice a day) to manage the lobar pneumonia. Because the patient was not considered immunocompromised by past medical history, physical examination, and routine laboratory test findings, we did not perform further immunologic function tests. He had received 13-valent pneumococcal conjugate vaccines and seasonal influenza vaccines, according to the recommended immunization schedule.
Fig. 1
Chest radiography shows right pleural effusion with consolidation in right upper lobe.
On the 1st day of hospitalization, generalized edema, oliguria, and microscopic hematuria worsened. As gram-positive cocci grew in the blood culture, we changed empirical parenteral antibiotics from ampicillin/sulbactam to vancomycin (360 mg, four times a day) and cefotaxime (1,200 mg, three times a day). On the 2nd day, there was no abnormality on the echocardiogram. In the analysis of pleural fluid, the WBC count was 32,000/mm3, the RBC count was 20,000/mm3, the protein level was 2.3 g/dL, and the lactate dehydrogenase level was 713 U/L. S. pneumoniae was detected in the antigen test but did not grow in the culture of pleural fluid. We did not perform any bacterial polymerase chain reaction (PCR) testing for pleural fluid. Serum C3, C4, and CH50 levels were decreased at 11 (normal value 90–180) mg/dL, 16 (normal value 16–47) mg/dL, and 10 (normal value 26–58) U/mL, respectively, whereas anti-streptolysin O (ASO) levels were increased at 550 (normal value 0–166) IU/mL.
On the 5th day, after cefotaxime has been administered for 5 days, cough and sputum began to improve, and his fever subsided. Penicillin-susceptible S. pneumoniae were finally identified from his blood culture; thus, we discontinued vancomycin and roxithromycin. His systolic BP increased to 139 mmHg, and his ASO level was >600 IU/mL on the 6th day. Microscopic hematuria increased to 50-99 RBCs/HPF, and the urine protein/creatinine ratio rose to 3.39. Respiratory virus real-time PCR testing of a nasopharyngeal swab and pleural fluid were both positive for influenza A virus.
On the 7th day of hospitalization, hematuria and proteinuria worsened, and his BP also increased; therefore, furosemide (20 mg, twice a day) was administered. Renal ultrasonography on the 9th day did not show any abnormal findings. Soon after the generalized edema disappeared, furosemide was changed to amlodipine (1.25 mg, once a day) and then to enalapril (3.75 mg, once a day) on the 13th day to control his high BP. The patient was discharged on the next day, and enalapril was continued for 6 months. Cefotaxime was administered intravenously for 14 days during hospitalization, and oral antibiotics were not prescribed at discharge. At discharge, the urine protein/creatinine ratio was 5.00, and gross hematuria persisted. Proteinuria was evaluated using protein/creatinine ratio. At 6 weeks after discharge, his serum C3 level increased to 91 mg/dL, and his urine protein/creatinine ratio decreased to 0.24 (
Table 1). Microscopic hematuria disappeared 14 weeks after discharge, and renal function was fully normalized 11 months after discharge. In addition, the
S. pneumoniae isolate was serologically typed by the Quellung reaction, at the research laboratory of the division of pediatric infectious diseases in Seoul National University Children's Hospital. The serotype was determined as 13.
Table 1
Laboratory findings of the patient on admission and OPD

|
ER #1 |
HD #3 |
HD #6 |
HD #9 |
HD #16 |
OPD #6 wks |
OPD #14 wks |
|
Serum |
|
|
|
|
|
|
|
|
C3 (mg/dL) |
|
11 |
20 |
27 |
45 |
91 |
103 |
|
C4 (mg/dL) |
|
16 |
|
|
|
21 |
20 |
|
ASO (IU/mL) |
|
550 |
>600 |
|
576 |
539 |
448 |
|
BUN (mg/dL) |
13 |
6 |
10 |
18 |
11 |
|
|
|
Cr (mg/dL) |
0.54 |
0.41 |
0.43 |
0.55 |
0.48 |
|
|
|
CRP (mg/dL) |
20.04 |
4.01 |
|
0.4 |
|
|
|
|
Protein (g/dL) |
6.0 |
6.1 |
|
7.4 |
|
8.0 |
7.4 |
|
Albumin (g/dL) |
2.7 |
2.2 |
3.1 |
3.6 |
4.2 |
4.2 |
4.5 |
|
Urine |
|
|
|
|
|
|
|
|
Albumin |
2+ |
+/− |
3+ |
2+ |
2+ |
+/− |
− |
|
Protein (mg/dL) |
|
18 |
424 |
224 |
403 |
14 |
6 |
|
Protein/Cr |
|
0.87 |
4.94 |
2.99 |
3.52 |
0.24 |
0.08 |
|
RBC/HPF |
10–19 |
10–19 |
≥100 |
>100 |
>100 |
50–99 |
1–4 |
DISCUSSION
We report a PIGN case associated with pneumococcal bacteremic pneumonia and influenza A virus infection in a 6-year-old Korean child. In the present case, PIGN developed 3 days after the onset of fever. After the use of antibiotics and antiviral agent as well as antihypertensive drugs and diuretics, the patient recovered completely from pneumonia and PIGN without long-term renal dysfunction.
A total of 11 cases of PIGN associated with pneumococcal infection have been reported in the literature; of these cases, eight occurred in children (
Table 2). Microscopic hematuria, proteinuria, and oliguria were present on admission in all pediatric cases, but hypertension was present in only 2 cases. Most cases (6/8) had pneumonia with pleural effusion or bacteremic pneumonia, as in this case. Nephritic symptoms, such as hematuria and proteinuria, developed within 1 week after the onset of fever in most cases (7/8). In this case, nephritic symptoms were observed in the hospital approximately 3 days after the onset of fever. Moreover, as seen in this case, the ASO titer was elevated in 5 of 8 previous cases. Among them, in two cases, pneumococcal polysaccharide and nephritis-associated plasmin receptor (NAPlr) were detected by using an immunofluorescence test.
23) This possibility was not investigated in the present study; however, confirming the deposition of pneumococcal polysaccharide or NAPlr through renal biopsy is helpful in the differential diagnosis.
Table 2
Clinical and epidemiological data of patients reported to have post-infectious glomerulonephritis developed with pneumococcal infection in the literature and this study

|
Reference |
Year |
Age (yr)/Sex |
Country |
Primary infection |
Interval*
|
Renal biopsy |
HTN |
ASO |
Serotype |
|
Hyman et al.2)
|
1975 |
4/F |
USA |
Lobar pneumonia, bacteremia |
2 days |
Deposition of C3 and pneumococcal type 14 antigen in mesangium and capillary walls |
− |
Elevated |
14 |
|
Schachter et al.5)
|
1987 |
5/M |
Israel |
Lobar pneumonia, bacteremia |
1 day |
NC |
+ |
Normal |
5 |
|
Wolach et al.6)
|
1990 |
3/M |
Israel |
Cervical lymphadenitis |
12 days |
NC |
+ |
Normal |
15 |
|
Phillips et al.7)
|
2005 |
6/F |
USA |
Segmental pneumonia, bacteremia |
7 days |
NC |
? |
NC |
7 |
|
Carceller et al.4)
|
2010 |
4/M |
Spain |
Lobar pneumonia, bacteremia |
3 days |
NC |
− |
Elevated |
17F |
|
Hibino et al.8)
|
2013 |
5/F |
Japan |
Bacteremia |
1 day |
NC |
− |
Elevated |
6C |
|
Ismail et al.9)
|
2014 |
4/M |
Malaysia |
Pneumonia with effusion, bacteremia |
6 days |
NC |
− |
Elevated |
NC |
|
Odaka et al.3)
|
2014 |
12/F |
Japan |
Lobar pneumonia with effusion, bacteremia |
6 days |
Deposition of C3c in the capillary loops |
− |
Elevated |
NC |
|
This study |
2017 |
6/M |
South Korea |
Lobar pneumonia, bacteremia |
3 days |
NC |
+ |
Elevated |
13 |
PSGN is caused by glomerular deposition of immune complexes or nephritis-inducing antigens, so the serum complement level is commonly decreased.
210) Additionally, in all previously reported PIGN cases associated with pneumococcal infections in children and in our case, serum C3 levels were decreased (
Table 2). In PSGN, the decrease in complement levels usually returns to normal approximately 8 weeks later,
11) but it normalized 6 weeks later in this case.
In most PIGNs associated with pneumococcal infection in children, nephritis develops within 1 week after fever onset,
2345789) whereas it develops within 1–2 weeks in PSGN cases. In patients with PIGN associated with pneumonia caused by other pathogens, including
Mycoplasma pneumoniae, the onset of nephritis occurs earlier, within 3 days.
41213) This may be due to the direct activation of the complement pathway in PIGN associated with bacteremia or pneumonia accompanying severe inflammatory reactions.
In this case, influenza A virus was simultaneously detected with pneumococcus as the etiology of pneumonia. Therefore, the possibility that influenza A virus caused PIGN cannot be excluded. However, only one case of AGN with influenza virus infection has been reported in children. A 14-year-old boy who complained of sore throat, flu-like symptoms, and gross hematuria was diagnosed with AGN and influenza A H1N1 infection.
14) On the other hand, a case of AGN with coinfection of influenza virus and pneumococcus or group A streptococcus has not been reported yet.
We report a case of PIGN associated with pneumococcal bacteremic pneumonia and influenza A virus infection in children. A urine test and measured BP should be considered for the early detection of PIGN in children with pneumococcal or influenza A virus infection when they present with nephritic symptoms.






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download