Dear Editor,
Severe mosquito bite allergy (SMBA; also known as hypersensitivity to mosquito bites), characterized by high fever and intense local skin symptoms following mosquito bites, is a very rare form of Epstein-Barr virus (EBV)-positive natural killer (NK)-cell lymphoproliferative disorder [
1]. Owing to its rarity and because the first signs primarily manifest as a cutaneous condition, many clinicians are likely to misdiagnose SMBA as common cellulitis [
1].
Although EBV infection and its oncogenic features are well known, SMBA has rarely been reported in Korea [
23]. In contrast to its name, SMBA is not an allergic disease but a hematologic disease, as mosquito saliva can trigger EBV reactivation and EBV-oncogene expression in latently infected NK cells [
45]. Life-threatening complications, such as NK/T-cell lymphoma or hemophagocytic lymphohistiocytosis (HLH), could be the most important effects throughout the extended clinical course of SMBA [
67]. We report the first Korean case of EBV-positive NK/T-cell lymphoma that progressed from SMBA, with coexistence of HLH. Two similar cases had been reported in Korea [
23]. One of these showed similar symptoms such as NK cell lymphocytosis and hemophagocytic syndrome, but it did not progress to lymphoma [
2]. The other case showed similar history as our patient, and the condition progressed to lymphoma; however, it was related to neither bone marrow (BM) involvement nor HLH [
3].
This case report was written in accordance with the Declaration of Helsinki (2013 revision) and approved by the Institutional Review Board of Asan Medical Center, Seoul, Korea. Written informed consent was obtained from the patient.
A 17-year-old boy had experienced waxing and waning edematous skin lesions whenever bitten by mosquitoes since 11 years of age, and those skin lesions had never been treated. As his symptoms were worsening, he visited a local dermatologist for his right upper arm mass lesion with necrosis in January 2018, and a skin excision was performed. However, the skin necrosis worsened. In February 2018, a skin biopsy of the right upper arm lesion was performed at Kyungpook National University Hospital, Daegu, Korea, which revealed NK/T-cell lymphoma with a background of T- and NK-cell type chronic active EBV infection.
In March 2018, he was admitted to Asan Medical Center. He showed high levels of EBV-viral capsid antigen IgG (396 U/mL) and EBV-early antigen IgG (82.0 U/mL) detected by chemiluminescence immunoassay (CLIA). Complete blood counts were as follows: Hb, 133 g/L; platelets, 118×10
9/L; and white blood cells, 2.1×10
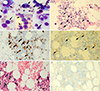
9/L, with 3% metamyelocytes, 47% segmented neutrophils, 35% lymphocytes, 12% monocytes, 2% eosinophils, and 1% reactive lymphocytes. At the same time, positron emission tomography-computed tomography revealed several hypermetabolic lesions in the right upper arm, lung, omentum, mesentery, and right iliac bone; these findings were compatible with those of NK/T-cell lymphoma. The BM study found no specific findings. In May 2018, the follow-up BM study revealed marked interstitial infiltration of neoplastic lymphocytes (
Fig. 1A & 1B) and frequent occurrence of hemophagocytic histiocytes. The BM biopsy revealed strong positivity for CD8 and CD56 immunohistochemical staining, and EBV in situ hybridization; CD4-positive T cells were markedly decreased (
Fig. 1C–1F). T-cell receptor (TCR) gene rearrangement study using BIOMED-2 PCR assays (InVivoScribe, San Diego, CA, USA) revealed TCR-γ rearrangements (
Fig. 2). BM involvement of NK/T-cell lymphoma was diagnosed.
Four cycles of SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase, etoposide) chemotherapy were initiated, and in October 2018, haplo-peripheral blood stem cell transplantation was done. The follow-up of BM study in June 2019 revealed no evidence of lymphoma involvement or hemophagocytic histiocytes. To monitor EBV activity in peripheral blood, regular follow-ups of EBV DNA quantification using real-time PCR were performed. The results fluctuated for eight months, ranging from negative to 3.01 log IU/mL.
In 2007, Asada, et al. [
5] reported the unique pathogenic mechanism of SMBA and revealed that the marked response of CD4+ T cells to mosquito saliva could induce the reactivation of latent EBV infection in NK cells. The dominant infiltration of CD4+ cells at mosquito bites in SMBA patients supports the aforementioned mechanism [
5]. Conversely, in 2013, Lee, et al. [
6] reported 14 SMBA episodes in four patients, demonstrating relatively decreased CD4+ cell proportions. In addition, they reported that as CD4+ cells may suppress EBV activation and inhibit the process of overwhelming HLH, decreased amounts of CD4+ cells could constitute a warning sign of BM failure, thus contributing to a worse prognosis [
6]. Thus, CD4+ cells may play a key role in SMBA pathogenesis. CD4 depletion could occur under the conditions of NK/T-cell lymphoma and hemophagocytic syndrome progression.
The aforementioned Korean cases in 2006 and 2010 further support the above-mentioned findings [
23]; the case without HLH had adequate CD4+ cells, whereas the case with HLH had decreased CD4+ cells. Our patient exhibited rare CD4+ cells in the BM biopsy with the absence of CD4+ cells in the skin. These findings are similar to those reported by Lee, et al. [
6], as our patient had HLH at the time of BM involvement of NK/T-cell lymphoma.
In conclusion, we report the first Korean case of EBV-positive NK/T-cell lymphoma that progressed from SMBA, with coexistence of HLH. In this case, marked neoplastic NK/T-cell proliferation with strong EBV positivity and decreased CD4+ T cells were noted. These findings suggest that CD4+ T cell depletion might induce EBV reactivation in NK cells, neoplastic proliferation of NK/T-cells, and occurrence of HLH. We recommend EBV DNA quantification and CD4-positive T cell measurement as a prognostic marker in the case of SMBA.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download