Abstract
Purpose
To evaluate the current use of intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) in patients with age-related macular degeneration (AMD).
Methods
We analyzed the number and medical costs of patients with AMD diagnosed by the National Health Insurance Corporation (2007–2016). We also analyzed the number and medical costs of such patients who received anti-VEGF treatment, and analyzed the frequency, period of use, and average medical cost of anti-VEGF use in AMD patients. Finally, we evaluated the use of anti-VEGF injections for new AMD patients.
Results
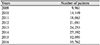
The number of patients with AMD was 236,158 in 2009 and 537,528 in 2016, which represented a 2.3-fold increase over 8 years. Of these, the number of patients undergoing anti-VEGF therapy increased steadily from 9,961 in 2009 to 35,762 in 2016. The mean number of cycles of ranibizumab or aflibercept per patient was 4.87 ± 3.37, and the mean interval between treatments was 2.89 months. On average, 6.2 injections were performed in the first year of diagnosis, and the frequency of use decreased with time, with an average of 1.2 cycles after 4 years of diagnosis. Among all AMD patients in 2016, the total medical cost of those treated with anti-VEGF was 76.9 billion won, and the average medical cost per person was 2,162,145 won.
Figures and Tables
Table 4
Number of patients of age-related macular degeneration received anti-vascular endothelial growth factor treatment (2009–2016)
References
1. Stefanini FR, Badaró E, Falabella P, et al. Anti-VEGF for the management of diabetic macular edema. J Immunol Res. 2014; 2014:632307.

2. Cheung GCM, Lai TYY, Gomi F, et al. Anti-VEGF therapy for neovascular AMD and polypoidal choroidal vasculopathy. Asia Pac J Ophthalmol (Phila). 2017; 6:527–534.

3. Mehta H, Tufail A, Daien V, et al. Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog Retin Eye Res. 2018; 65:127–146.

4. Campa C, Alivernini G, Bolletta E, et al. Anti-VEGF therapy for retinal vein occlusions. Curr Drug Targets. 2016; 17:328–336.

5. Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008; 358:2606–2617.

6. Ashraf M, Souka AAR. Aflibercept in age-related macular degeneration: evaluating its role as a primary therapeutic option. Eye (Lond). 2017; 31:1523–1536.

7. Villegas VM, Aranguren LA, Kovach JL, et al. Current advances in the treatment of neovascular age-related macular degeneration. Expert Opin Drug Deliv. 2017; 14:273–282.

8. Zhang Y, Chioreso C, Schweizer ML, Abràmoff MD. Effects of aflibercept for neovascular age-related macular degeneration: a systematic review and meta-analysis of observational comparative studies. Invest Ophthalmol Vis Sci. 2017; 58:5616–5627.
9. Gemenetzi M, Patel PJ. A systematic review of the treat and extend treatment regimen with anti-VEGF agents for neovascular age-related macular degeneration. Ophthalmol Ther. 2017; 6:79–92.

10. Al-Zamil WM, Yassin SA. Recent developments in age-related macular degeneration: a review. Clin Interv Aging. 2017; 12:1313–1330.

11. La TY, Cho E, Kim EC, et al. Prevalence and risk factors for age-related macular degeneration: Korean National Health and Nutrition Examination Survey 2008-2011. Curr Eye Res. 2014; 39:1232–1239.

12. Park SJ, Cho SC, Choi NK, et al. Age, sex, and time-specific trends in surgical approaches for rhegmatogenous retinal detachment: a nationwide, population-based study using the national claim registry. Retina. 2017; 37:2326–2333.
13. Ha YC, Kim HY, Jang S, et al. Economic burden of osteoporosis in South Korea: claim data of the national health insurance service from 2008 to 2011. Calcif Tissue Int. 2017; 101:623–630.

14. Kim S, Ahn H, Shin SA, et al. Trends of thromboprophylaxis and complications after major lower limb orthopaedic surgeries in Korea: National Health Insurance Claim Data. Thromb Res. 2017; 155:48–52.

15. Brown GC, Brown MM, Sharma S, et al. The burden of age-related macular degeneration: a value-based medicine analysis. Trans Am Ophthalmol Soc. 2005; 103:173–186. discussion 184-6.
16. Brown MM, Brown GC, Stein JD, et al. Age-related macular degeneration: economic burden and value-based medicine analysis. Can J Ophthalmol. 2005; 40:277–287.

17. Schmier JK, Jones ML, Halpern MT. The burden of age-related macular degeneration. Pharmacoeconomics. 2006; 24:319–334.

18. Pożarowska D, Pożarowski P. The era of anti-vascular endothelial growth factor (VEGF) drugs in ophthalmology, VEGF and anti-VEGF therapy. Cent Eur J Immunol. 2016; 41:311–316.

19. Lorence DP, Spink A. Regional variation in medical systems data: influences on upcoding. J Med Syst. 2002; 26:369–381.
20. Ziemssen F, Grisanti S, Bartz-Schmidt KU, Spitzer MS. Off-label use of bevacizumab for the treatment of age-related macular degeneration: what is the evidence. Drugs Aging. 2009; 26:295–320.




 PDF
PDF ePub
ePub Citation
Citation Print
Print










 XML Download
XML Download