Abstract
Purpose
Materials and Methods
Results
Figures and Tables
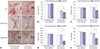 | Fig. 1Relaxin (RLX) expression reduces the size, color index, and pliability of pig scars. (A) Scars were created on the backs of pigs. The area of scars decreased, and the color improved at 50 days after the injection of relaxin-expressing Ad loaded in alginate gel (gel/Ad-RLX). (B) Scar size, (C) erythema index values, (D) melanin index values, and (E) pliability were significantly reduced (†p<0.05) compared to those of control groups [gel or LacZ-expressing Ad loaded in alginate gel (gel/Ad-LacZ)]. Data are expressed as a mean±standard error of the mean. |
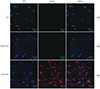 | Fig. 2Immunofluorescence staining to evaluate the effects of relaxin on scar reduction in pig scar tissues. Increased expression of relaxin was detected after treatment with relaxin-expressing Ad loaded in alginate gel (gel/Ad-RLX). In contrast, control groups [gel or LacZ-expressing Ad loaded in alginate gel (gel/Ad-LacZ)] showed lower levels of relaxin. Nuclei were visualized by DAPI (4',6-diamidino-2-phenylindole) staining; original magnification, ×400; scale bar=20 µm. |
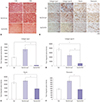 | Fig. 3Picrosirius red staining and immunohistochemical staining for collagen type-I, collagen type-III, elastin, and fibronectin in pig scar tissues. (A) Dense and coarse collagen fibers were replaced with closely packed collagen fibers after pig scar tissues were treated with gel/Ad-RLX; original magnification, ×100 and ×200; scale bar=500 µm and 200 µm. (B) The expression levels of the major extracellular matrix components collagen type-I, collagen type-III, elastin, and fibronectin were lower in pig scar tissues treated with gel/Ad-RLX than those in pig tissues treated with gel or gel/Ad-LacZ; original magnification, ×400; scale bar=100 µm. Semi-quantitative image analyses revealed that (C) collagen type-I, (D) collagen type-III, (E) elastin, and (F) fibronectin were significantly decreased in pig tissues treated with gel/Ad-RLX, compared to those in pig tissues treated with control virus (*p<0.01). Data are expressed as a mean±standard error of the mean. |
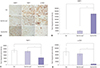 | Fig. 4Immunohistochemical staining for matrix metalloproteinase-1 (MMP-1), tissue inhibitor of metalloproteinase-1 (TIMP-1), and alpha-smooth muscle actin (α-SMA) in pig scar tissues. (A) Treatment with gel/Ad-RLX markedly increased MMP-1 levels and decreased TIMP-1 and α-SMA levels in pig scar tissues; original magnification, ×400; scale bar=100 µm. Semi-quantitative image analyses revealed that (B) MMP-1 was significantly increased in pig tissues treated with gel/Ad-RLX versus that of tissues treated with control virus (*p<0.01). (C) TIMP-1 and (D) α-SMA levels were significantly decreased in pig tissues treated with gel/Ad-RLX, compared to those with control virus (†p<0.05). Data are expressed as mean±standard error of the mean. |
 | Fig. 5Relaxin-expressing Ad decreases transforming growth factor-β1 (TGF-β1) and increases TGF-β3 mRNA levels in pig scar tissues. (A) Quantitative analyses by qRT-PCR indicated that TGF-β1 mRNA levels were significantly decreased (†p<0.05) in scar tissues treated with gel/Ad-RLX, compared with those of tissue treated with control virus. (B) In contrast, TGF-β3 mRNA was significantly increased (†p<0.05) by relaxin overexpression in pig scar tissues. Data are expressed as a mean±standard error of the mean. |
ACKNOWLEDGEMENTS
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: Won Jai Lee and Chae-Ok Yun.
Data curation: Eunhye Kang.
Formal analysis: Hyo Min Ahn.
Funding acquisition: Chae-Ok Yun.
Investigation: In Sik Yun.
Project administration: Yong Oock Kim.
Supervision: Dong Kyun Rah and Chae-Ok Yun.
Validation: Won Jai Lee.
Writing—original draft: In Sik Yun.
Writing—review & editing: Tai Suk Roh.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download