INTRODUCTION
Peyronie's disease (PD), also known as “induratio penis plastica”, is a superficial fibrous condition of the penis characterized by the presence of fibrotic plaques in the tunica albuginea causing pain, abnormal curvature and erectile dysfunction (ED) [
12345]. Moreover, it could be considered as a psychosocial disease since it affects the sexual life of the patient [
678]. It typically affects males between the ages of 45 and 60 years [
9]. Indeed, as described in literature, the mean reported age at disease presentation was 53.5 years [
10]. Once thought to be rare, PD has a reported prevalence of up to 13.1% in adult men [
11]. Nevertheless, the etiology of PD is not entirely understood, but is likely related to trauma followed by abnormal wound healing. Interestingly, the predisposition to fibrosis and abnormal wound healing might have a genetic basis in some affected men. It is important to note that most treatment methods have little evidence in the literature supporting their efficacy. Of the medications currently used, Collagenase Clostridium Hystoliticum (CCH) is the only Food and Drug Administration-approved drug for treatment of PD improving penile curvature and symptom bother. In this scenario, extracorporeal shockwave therapy (ESWT) represents an emergent minimally invasive approach. Despite several theories addressing the effect of lithotripters [
12], these cannot be directly applied to understand the effect of shockwave on soft tissues. A regenerative potential of shockwave on several organs has been suggested, contrary to lithotripsy, which has a destructive impact on a urinary stone. In particular, ESWT may act by mechanical stimulation of cells [
13], by stimulating synthesis of nitric oxide (NO), vascular endothelial growth factor (VEGF) and inducing angiogenesis in the penile cavernous tissue.
The aim of this single-arm was to evaluate the efficacy and safety of ESWT treatment in a large cohort of patients affected by PD.
Go to :

MATERIALS AND METHODS
From January 2016 to January 2018, we enrolled 325 consecutive patients affected by PD in a multi-center single-arm clinical study. The study has been conducted according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [
14].
Patients with ≥18 years, active phase, and plaques and/or pain at erection and/or deviation were included in the study.
Patients who have received previous intralesional therapy, oral drugs and patients without pain were excluded from the study.
All patients presented single plaque, evaluated at first by physical examination and subsequently by penile Doppler ultrasounds. All participants were adequately counselled and signed a detailed consent form prior to treatment and agreed to have their data anonymously utilized. All patients received ESWT (DUOLITH® SD1 ultra; STORZ MEDICAL AG, Tägerwilen, Switzerland). The treatment was performed perpendicular on the penile shaft exactly on the top of the plaque with 1,500 pulses in the right side of the plaque and then 1,500 in the left side (3,000 hit at 0.25 mJ/mm2). Each patient had 6 treatments, one treatment per week for six weeks.
Penile curvature was measured by a goniometer after intracavernosal drug-induces erection using Alprostadil (Viridal®; Schwarz Pharma, Monheim, Germany) and penile length during erection. Plaque size was measured with a ruler in cm
2 using the ultrasound. Sexual function was assessed by the international index of erectile function (IIEF-15). Severity of ED was classified as severe (IIEF-15 ≤10), moderate (IIEF-15 between 11 and 16), or mild (IIEF-15 between 17 and 25). The IIEF-15 sub-scores were assessed, including IIEF-erectile function (IIEF-EF), IIEF-orgasmic function (IIEF-OF), IIEF-sexual desire (IIEF-SD), IIEF-intercourse satisfaction (IIEF-IS), and IIEF-overall satisfaction (IIEF-OS) [
15]. Moreover, to measure the impact and severity of PD symptoms, patients were asked to fill out the PD questionnaire (PDQ). PDQ is a 15-question self-reported survey that measures the impact and severity of PD symptoms in three domains: psychological and physical symptoms (PS symptom severity score; PDQ question 1–6), penile pain (penile pain score; PDQ question 7–9), and symptom bother (bother score; PDQ question 10–15). All patients completed the PDQ [
16].
The results were evaluated at baseline and 3 months after the treatment. No other therapy for PD or ED was continued during this period. Phosphodiesterase 5 inhibitors on demand (PDE-5i) were discontinued about 3 weeks before the ESWT. In fact, as previously reported, PDE-5i may act by the elevation of cyclic guanosine monophosphate levels and activation of protein kinase G, which are involved in cell apoptosis and reducing collagen synthesis [
81718].
The patients continued to have their regular lifestyle, sexual status, and other routine medications. Treatment emergent adverse events (TEAEs) were collected using self-reported events. The relation of adverse events to treatment was evaluated by the investigator based on the temporal relation to treatment and the likelihood of an alternative aetiology.
1. Ethics statement
study was conducted in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. Each patient signed a written informed consent to collect data.
2. Statistical analysis
Continuous variables are presented as median and interquartile range (IQR) and were compared by the Student independent t-test or the Mann-Whitney U-test based on their normal or not-normal distribution, respectively (normality of variables' distribution was tested by the Kolmogorov-Smirnov test). Categorical variables were tested with the chi-square test.
All statistical analyses were completed using Stata software, ver. 14 (StataCorp., College Station, TX, USA). For all statistical comparisons, a significance level of p<0.05 was considered to show differences between the groups.
Go to :

RESULTS
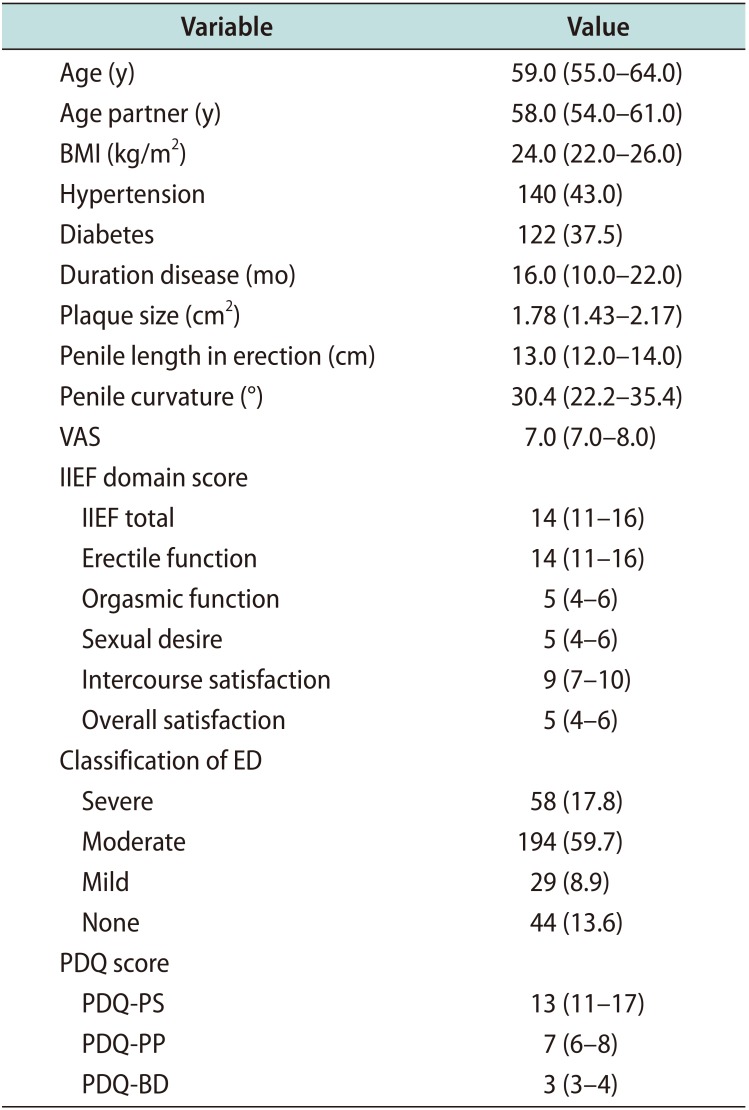
Table 1 lists the baseline characteristics of the study cohort. There were no dropouts, and the patients came for regular sessions and follow-up to complete questionnaires. Overall, median age of 59.0 years (IQR, 55.0–64.0 years), median disease duration was 16.0 months (IQR: 10.0–22.0 months), median plaque size was 1.78 cm
2 (IQR, 1.43–2.17 cm
2), median penile length in erection was 13.0 cm, median penile curvature was 30.4° (IQR, 22.2°–35.4°), median IIEF-EF was 14 (IQR, 11–16) and median PDQ was 13 (IQR, 11–17). The most common plaque site was in penile dorsum. As concerning comorbidities, 122 (37.5%) had diabetes and 140 (43.0%) had hypertension.
Table 1
Baseline characteristics of the population (n=325)
|
Variable |
Value |
|
Age (y) |
59.0 (55.0–64.0) |
|
Age partner (y) |
58.0 (54.0–61.0) |
|
BMI (kg/m2) |
24.0 (22.0–26.0) |
|
Hypertension |
140 (43.0) |
|
Diabetes |
122 (37.5) |
|
Duration disease (mo) |
16.0 (10.0–22.0) |
|
Plaque size (cm2) |
1.78 (1.43–2.17) |
|
Penile length in erection (cm) |
13.0 (12.0–14.0) |
|
Penile curvature (°) |
30.4 (22.2–35.4) |
|
VAS |
7.0 (7.0–8.0) |
|
IIEF domain score |
|
|
IIEF total |
14 (11–16) |
|
Erectile function |
14 (11–16) |
|
Orgasmic function |
5 (4–6) |
|
Sexual desire |
5 (4–6) |
|
Intercourse satisfaction |
9 (7–10) |
|
Overall satisfaction |
5 (4–6) |
|
Classification of ED |
|
|
Severe |
58 (17.8) |
|
Moderate |
194 (59.7) |
|
Mild |
29 (8.9) |
|
None |
44 (13.6) |
|
PDQ score |
|
|
PDQ-PS |
13 (11–17) |
|
PDQ-PP |
7 (6–8) |
|
PDQ-BD |
3 (3–4) |

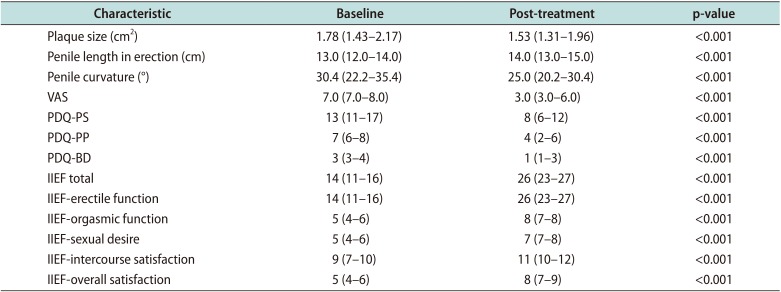
After treatment, we observed a significant reduction of plaque size to 1.53 cm2 (IQR, 1.31–1.96 cm2) (p<0.001) of penile length to 14.0 cm (IQR, 13.0–15.0) (p<0.001). We also found a decrease of penile curvature to 25.0° (IQR, 20.2°–30.4°) (p<0.001) and in visual analogue scale (VAS) from 7.0 (IQR, 7.0–8.0) to 3.0 (IQR, 3.0–6.0) (p<0.001).
As concerning sexual function, the improvement in IIEF-15 was marked by an overall improvement in all the domains of IIEF: the median EF score, the median OF score, the median SD score, the median IS score, the median OS score (
Table 2) (all p<0.001). There was an improvement in each of PDQ domains, clinically significant (p<0.001) (
Table 2,
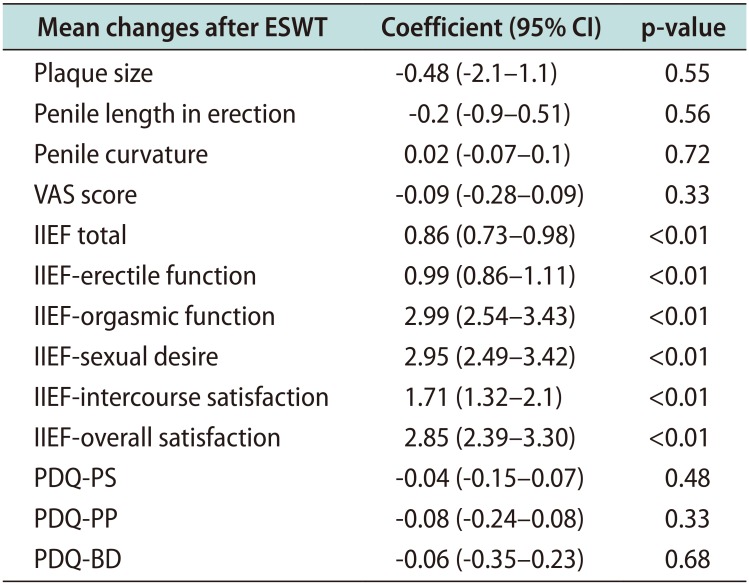
Fig. 1). We also evaluated the correlation between duration of disease and mean difference of sexual questionnaires after treatment. We performed linear regression between VAS mean difference and IIEF-15 mean difference and we did not demonstrate significant association (r=-0.10; p=0.40). The results are shown in
Table 3. As it is possible to highlight, a longer duration of the disease correlates with a statistically significant improvement of sexual function.
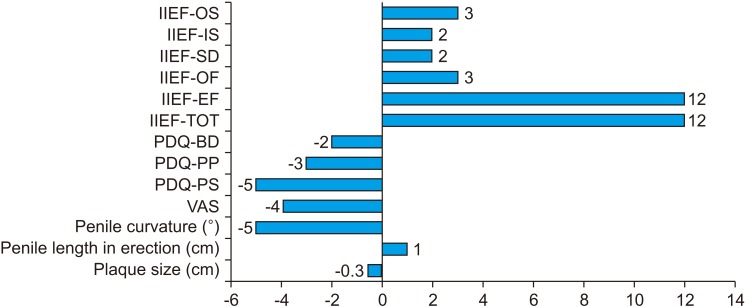 | Fig. 1Median difference between questionnaires before and after treatment. IIEF: international index of erectile function, OS: overall satisfaction, IS: intercourse satisfaction, SD: sexual desire, OF: orgasmic function, EF: erectile function, TOT: tatal, PDQ: Peyronie's disease-questionnaire, BD: bother domain, PP: penile pain, PS: physical symptom, VAS: visual analogue scale.
|
Table 2
Characteristics of the patients after treatment
|
Characteristic |
Baseline |
Post-treatment |
p-value |
|
Plaque size (cm2) |
1.78 (1.43–2.17) |
1.53 (1.31–1.96) |
<0.001 |
|
Penile length in erection (cm) |
13.0 (12.0–14.0) |
14.0 (13.0–15.0) |
<0.001 |
|
Penile curvature (°) |
30.4 (22.2–35.4) |
25.0 (20.2–30.4) |
<0.001 |
|
VAS |
7.0 (7.0–8.0) |
3.0 (3.0–6.0) |
<0.001 |
|
PDQ-PS |
13 (11–17) |
8 (6–12) |
<0.001 |
|
PDQ-PP |
7 (6–8) |
4 (2–6) |
<0.001 |
|
PDQ-BD |
3 (3–4) |
1 (1–3) |
<0.001 |
|
IIEF total |
14 (11–16) |
26 (23–27) |
<0.001 |
|
IIEF-erectile function |
14 (11–16) |
26 (23–27) |
<0.001 |
|
IIEF-orgasmic function |
5 (4–6) |
8 (7–8) |
<0.001 |
|
IIEF-sexual desire |
5 (4–6) |
7 (7–8) |
<0.001 |
|
IIEF-intercourse satisfaction |
9 (7–10) |
11 (10–12) |
<0.001 |
|
IIEF-overall satisfaction |
5 (4–6) |
8 (7–9) |
<0.001 |

Table 3
Linear regression analysis between duration of the disease and mean difference of sexual questionnaires after treatment
|
Mean changes after ESWT |
Coefficient (95% CI) |
p-value |
|
Plaque size |
-0.48 (-2.1–1.1) |
0.55 |
|
Penile length in erection |
-0.2 (-0.9–0.51) |
0.56 |
|
Penile curvature |
0.02 (-0.07–0.1) |
0.72 |
|
VAS score |
-0.09 (-0.28–0.09) |
0.33 |
|
IIEF total |
0.86 (0.73–0.98) |
<0.01 |
|
IIEF-erectile function |
0.99 (0.86–1.11) |
<0.01 |
|
IIEF-orgasmic function |
2.99 (2.54–3.43) |
<0.01 |
|
IIEF-sexual desire |
2.95 (2.49–3.42) |
<0.01 |
|
IIEF-intercourse satisfaction |
1.71 (1.32–2.1) |
<0.01 |
|
IIEF-overall satisfaction |
2.85 (2.39–3.30) |
<0.01 |
|
PDQ-PS |
-0.04 (-0.15–0.07) |
0.48 |
|
PDQ-PP |
-0.08 (-0.24–0.08) |
0.33 |
|
PDQ-BD |
-0.06 (-0.35–0.23) |
0.68 |

As concerning the presence of diabetes in our cohort, we verified the putative changes of all variables according to the presence of diabetes.
We observed a significant median increase of IIEF-IS (1.0 vs. 2.0; p=0.04) in diabetes while significant decrease of VAS in non-diabetics (−0.8 vs. −0.4 [standard deviation, 1.33]; p=0.02).
For the other variables, no significant changes were observed for PDQ-PS (p=0.48), PDQ-penile pain (p=0.33), PDQ-bother domain (p=0.68), plaque size (p=0.55), and penile curvature (p=0.72).
We performed similar analysis for other risk factors, like hypertension or body mass index but we did not find significant differences for all variables. No TEAEs following ESWT were recorded. None of the patients in our study reported any allergic reaction to the gel used for ESWT.
Go to :

DISCUSSION
In the present study, we showed that ESWT treatment is able to reduce objectively the severity of the disease and to improve sexual function in a group of patients suffering from PD.
Recently, ESWT is gaining interest for the treatment of PD and ED thanks to its mini-invasive characteristics. Nevertheless, doubts still exist regarding the modality of application and its biological effects. Meta-analysis by Hauck et al [
19] shows that decrease in plaque size was reported in 0% to 68% of cases, decrease in curvature reported as no significant findings to 74% of cases and decrease in penile pain in 56% to 100%. Sexual function, typically not clearly defined, improved in 12% to 80%. These different results could be explained by the different groups of patients, modes of evaluation, techniques and study designs. But it should be noted that accurate measurement of plaque size is difficult with any imaging or mechanical modality.
Moreover, results regarding plaques are difficult to interpret because plaque size reduction is not really a treatment aim, as penile deviation is the most troublesome symptom. In a randomized clinical trial from Palmieri et al [
20], authors assumed that ESWT may have a protective effect on disease progression by stabilizing deviation and plaques. In another in a randomized clinical trial [
21] there are not reported benefit respect to penile curvature, on the contrary to our results, which shows significant improvement in the subjective assessment of the penile curvature pre- and post-treatment (p<0.001). A meta-analysis from Gao et al [
22] showed that ESWT could significantly increase the percentage of men with lessening of penile plaques (odds ratio [OR], 2.07), relief of pain (OR, 4.46), complete remission of pain (OR, 5.86), and slight differences for penile curvature (OR, 1.88; p=0.06), while no significant differences for sexual function (OR, 2.22) between ESWT and placebo groups.
However, in this meta-analysis outcomes were analyzed dichotomously and not continuous while in our study they were analyzed continuously. In fact, we assessed penile curvature in the erect state, with a slight improvement from median 30.4° (IQR: 22.2°–35.4°) at baseline to 25.0° (IQR: 20.2°–30.4°) post-treatment.
Decreases in deviation in literature vary between 21% and 74%. Hauck et al [
23] found a significant reduction in the subgroup of patients with deviation of 31° to 60° before ESWT. Mean deviation decreased from 45.7° to 38.5° in this group. Like this, Palmieri et al [
20] noted a significant reduction in deviation between the ESWT and placebo group (31°
vs. 27°, respectively).
It is important to underline, that the impact of ESWT on PD is basically determined by pain relief. In fact, in our study, we showed to decreased from 7 to 4 (VAS) but this effect did not influence IIEF-15 changes after treatment. Moreover, we also found that a longer duration of the disease was associated with statistically significant improvement of sexual function.
Many other previous studies have tried to attempt predictors of response in patients with PD [
2425], however data regarding outcomes after ESWT are lacking.
A previous study from Cocci et al [
6] in patients receiving CCH demonstrated that patients with longer PD duration, greater baseline penile curvature and basal and dorsal plaque location had a greater chance of treatment success in terms of penile curvature.
Although our results are not comparable to these previous, we postulate that the longer the duration of the disease the most stable is PD. This may influence both restoring the sexual function considering both stabilization of curvature and also better psychological impact for the patients.
The mechanism of ESWT's effect in PD is unclear, although satisfactory results were obtained from a lot of studies. ESWT may directly damage and remodel penile plaque. In fact, local circulation may be increased in consequence of generating heat caused by ESWT, which can result in an inflammatory reaction followed by increased macrophage activity, leading to plaque lysis and resorption [
26]. However, several discrepancies are present in the literature data that failed to drive specific recommendation about the clinical efficacy of ESWT.
Previous literature data have reported that ESWT has a positive short-term clinical and physiological effect on the EF. In the randomized, double-blind, sham controlled study done by Vardi et al [
27] ESWT has been shown to have a beneficial effect on EF in men with PD. Protocol provided to receive 12 sessions of low intensity ESWT or sham therapy. Instead, open-label single-arm prospective study by Gruenwald et al [
28] shows that ESWT has a physiologic effect on the erectile mechanism and to improve IIEF-ED. The protocol was two treatment sessions per week for 3 weeks, which were repeated after a 3-week no-treatment interval. In the prospective, randomized, double-blind, placebo-controlled study by Palmieri et al [
20] it is shown that after 12 weeks VAS score, IIEF score ameliorated significantly in patients receiving ESWT. After 24 weeks plaque size and curvature degree were significantly reduced. Indeed, Palmieri et al [
20] evaluated that mean plaque size and mean curvature degree were significantly higher in the placebo group when compared with both baseline and ESWT values. Treatment sessions were performed once weekly for four consecutive weeks. In agreement with the results in studies done by Vardi et al [
27], Gruenwald et al [
28] and Palmieri et al [
20], we have found improvement in all the domains of IIEF and in the sexual function, reduction in VAS score, plaque size and penile curvature. Regarding VAS score, in our study it was reduced by 4 points after treatment compared to baseline. In fact, the median VAS baseline was 7.0 (7.0–8.0) which improved to 3.0 (3.0–6.0) post-treatment. This shows statistically significant (p<0.001) improvement in the pain score of patients' post-treatment.
ESWT may act by mechanical stimulation of cells [
1329], by stimulating synthesis of NO, VEGF and inducing angiogenesis in the penile cavernous tissue. Although this is out of the scope of our study, these premises could arise some questions about its role in patients with diabetes or hypertension.
Although specific associations between diabetes, hypertension and PD are difficult to arise, many reports found positive link [
3031]. The diabetes might condition the gravity of the disease because it would worsen the micro-circulation of penis and determine a considerable fibrotic process due to disease.
Blood hypertension is shown to be differently associated to PD, even though the etiological and pathophysiological factors of this association is unknown. A case-cohort study from Pavone et al [
32] showed that among patients with blood hypertension PD was diagnosed in 69 patients (44%) and that among patients with normal blood pressure it was diagnosed in 28 patients (23%) (p<0.001).
Our favorable results, in fact, maybe also justified by the high prevalence of diabetes in our cohort, that can benefit from the ESWT activity in terms of improving micro-circulation.
We would also point out that our favorable findings about plaque size and penile curvature improvement are difficult to transfer into clinical practice. In fact, patients may not be aware of such improvement. On the contrary, pain and sexual function increase represent the main patient reported outcomes after ESWT.
For these reasons, we support translation of our results into clinical practice for several aspects. ESWT represents a valuable treatment for PD patients, ameliorating penile curvature, EF, plaque size, and pain resolution. Although we are cannot did not compare different energy source delivering, our protocol was demonstrated to be reliable and efficient.
Some limitations of this study should be addressed. This is a single arm non-randomized clinical trial to evaluate the benefits of ESWT in the management of PD. Furthermore, the open label design without control may have influenced clinical outcomes. Finally, the 3 weeks of dropout from PDE-5i should be considered when evaluating our clinical outcomes. The study is feasible for the availability of the ESWT machine and because are not necessarily special skills. However, more randomized controlled trials studies should be performed to make ESWT a valid procedure. A longer follow-up period would also serve to gain a better understanding of the potential limitations of the therapy.
Go to :









 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download