Abstract
Figures and Tables
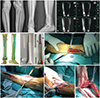 | Fig. 1Preoperative examination, design, and construction of the prosthesis using three-dimensional (3D)-printing technology. (A) Radiography of diseased tibia. (B) Magnetic resonance imaging of the diseased tibia and fibula. (C) 3D prosthesis design in a computer. (D) 3D-printing resinous model and titanium alloy prosthesis; the latter has a porous printed structure (0.25–0.40 cm thickness) of the bone trabecula on the peripheral surface of the stem, collar, and ankle mortise (red arrowheads). The prosthesis was made with 3D printing in one step with its porous coating. (E) Incision I. (F) Incision II and incision III. (G) Installation of the proximal end of the prosthesis. (H) Installation of the distal end of the prosthesis. |
 | Fig. 2Postoperative review. (A) Good prosthesis position and lower limb force line in full-length radiography images at 3 months after the operation. (B) At 12 months after the operation, radiography images show no obvious progressive radiolucent zones around the prosthesis stem and metal ankle mortise, as well as several new bone bridge spanning the bone-prosthesis junction outside the cortex and prosthesis (red arrowheads). (C) Computed tomography scan shows bone growth into the porous surface of the prosthetic stem and ankle mortise (red arrowheads) and into the porous surface of the prosthetic collar and the front of metal ankle mortise (from the extracortical bone bridge, green arrowheads) at 9 months after the operation. (D) Lower extremity movement of the patient at 9 months after the operation. |
Table 1
MSTS 93 Scores of Surgical Limbs at 6 and 12 Months after Operation

| Time | Pain | Function | Emotional acceptance | Support | Walking | Gait |
|---|---|---|---|---|---|---|
| 6 months after operation | 5 | 3 | 4 | 3* | 3 | 1† |
| 12 months after operation | 5 | 3 | 5 | 5 | 5 | 3 |
The MSTS 1993 score includes pain, function, emotional acceptance, supports, walking ability, and gait, and ranges from 0 to 30. Excellent: 24–30 points, good: 18–23 points, fair:12–17 points, poor: 12 points or less. The final MSTS 1993 score is presented as a percentage.
*Brace using; †Major cosmetic.
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: Dehong Feng.
Data curation: Yu Guo and Ling Wang.
Formal analysis: Ling Wang and Yu Guo.
Funding acquisition: Dehong Feng.
Investigation: Junshan He and Chenyu Zhang.
Methodology: Ling Wang and Xiaofeng Gu.
Project administration: Dehong Feng and Xiaofeng Gu.
Resources: Dehong Feng, Junshan He, and Xiaofeng Gu.
Software: Chenyu Zhang and Yu Guo.
Supervision: Ling Wang and Xiaofeng Gu.
Validation: Chenyu Zhang and Ling Wang.
Visualization: Junshan He.
Writing—original draft: Dehong Feng and Junshan He.
Writing—review & editing: Dehong Feng.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download