INTRODUCTION
The onset of coronary artery aneurysm (CAA) after drug-eluting stent (DES) implantation is uncommon, with an incidence ranging from 0.8% to 1.3%. However, associated adverse clinical events may present as a result of DES thrombosis and restenosis.
1234 Furthermore, the occurrence of DES thrombosis appears to be particularly high in patients with CAA who discontinued dual-antiplatelet therapy.
4 Importantly, most previous studies have been conducted involving patients treated with first-generation DES implantation alone and, although concerns about the development of CAA after DES implantation have decreased after the introduction of second-generation DESs, occurrences of CAA even after second-generation DES implantation have been reported in recent case reports.
567 At this time, to our knowledge, the incidence of CAA after second-generation DES implantation has not been evaluated, and even more concerning, the clinical implications of angiographic CAA after second-generation DES placement remain uncertain. Therefore, we sought to evaluate the incidence and predictors of CAAs after second-generation DES implantation and to compare clinical outcomes in these patients with those of patients with CAAs after first-generation DES implantation using data from our CAA registry.
4
MATERIALS AND METHODS
Study population
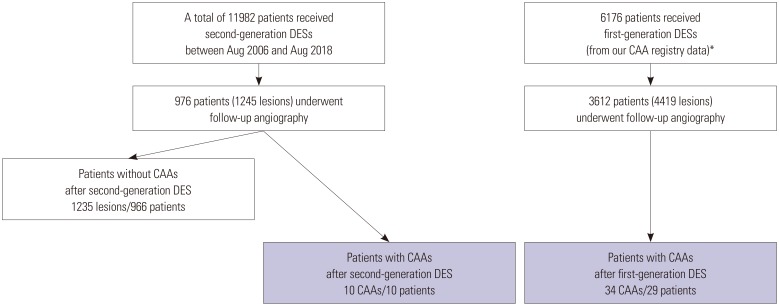
This study was a single-center, retrospective, observational study. Between August 2006 and August 2018, a total of 11982 consecutive patients underwent second-generation DES implantation (
Fig. 1). Of these 11982 patients, 976 patients (8.1%) with 1245 lesions underwent follow-up angiography within 2 years of second-generation DES placement, including 816 patients (83.6%) for routine follow-up without any symptoms and 160 patients (16.4%) for the evaluation of newly-developed symptoms, respectively. To compare with CAAs after first-generation DES implantation, angiographic data and clinical outcomes of 34 first-generation DES-related CAAs were obtained from our previous CAA registry data (
Fig. 1).
4 Briefly, these registry data from four independent referral hospitals in South Korea included 3612 consecutive patients (4419 lesions) with late angiographic follow up after first-generation DES implantation, which represented 58.4% of the larger initial population. In this subpopulation, 34 CAAs (0.77%) in 29 patients (0.80%) were detected during late follow-up angiography.
4 This study was approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System (No.: 4-2018-0759).
Intervention procedure
Stent implantation was performed according to current standard techniques and medical guidelines. A total of 1245 lesions were treated with second-generation DESs: 425 everolimuseluting (Xience, Abbot Vascular, Santa Clara, CA, USA; and Promus, Boston Scientific, Marlborough, MA, USA), 399 zotarolimus-eluting (Endeavor Resolute, Medtronic, Minneapolis, MN, USA), 89 sirolimus-eluting (Orsiro, Biotronik, Berlin, Germany), and 332 biolimus-eluting (BioMatrix, Biosensors International, Singapore; and Nobori, Terumo, Tokyo, Japan) stents were used.
All patients were given 300 mg of aspirin in addition to 300 mg to 600 mg of clopidogrel, 180 mg of ticagrelor, or 60 mg of prasugrel before their procedure and were maintained on dual-antiplatelet therapy after DES implantation for at least 6 months. During the intervention, unfractionated heparin was administered to maintain an activated clotting time of more than 250 seconds. Specific details of the intervention, such as lesion predilatation, poststent dilation, and the application of mechanical support or concomitant medication, were left to the discretion of the operator.
Definition and clinical follow up
A dilatation of a major epicardial, stented coronary artery that exceeded the diameter of the normal adjacent reference vessel by 1.5 times that was closely related to the underlying DES or its edges and was not present immediately after the procedure was defined as CAA.
14 According to a previous study,
4 CAAs can be classified into three different types: (1) ectatic type, a diffuse aneurysmal dilatation of the coronary artery that involves more than 50% of the stent length; (2) fusiform type, a spindle-shaped dilatation (along the axis of a vessel with at least twice the diameter of the transverse dimension); and (3) saccular type, a single or multiple spherical-shaped dilatation (the transverse dimension is usually greater than the longitudinal dimension).
4
A major adverse cardiac event (MACE) was defined as a composite of cardiac death, myocardial infarction, and stent thrombosis. All deaths were considered cardiac deaths unless a definite noncardiac cause could be established. After hospital discharge, myocardial infarction was defined as the presence of consistent clinical symptoms, electrocardiographic changes, or abnormal imaging findings in combination with a creatine kinase myocardial band fraction increase of greater than the upper normal limit or an increase in troponin T or troponin I to more than the 99th percentile of the upper normal limit.
89 The Academic Research Consortium definition of definitive or probable stent thrombosis was used.
48
Statistical analysis
Continuous variables are reported as a mean±standard deviation or median (interquartile range) as appropriate and were compared using Student's t-test or the Mann-Whitney U test. Categorical variables are reported as either a number or number (percentage) and were compared using Fisher's exact test or the chi-squared test. Adverse event rates were compared using a log-rank test. Findings were considered significant at p<0.05. All statistical analyses were performed using the Statistical Package for the Social Sciences, version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
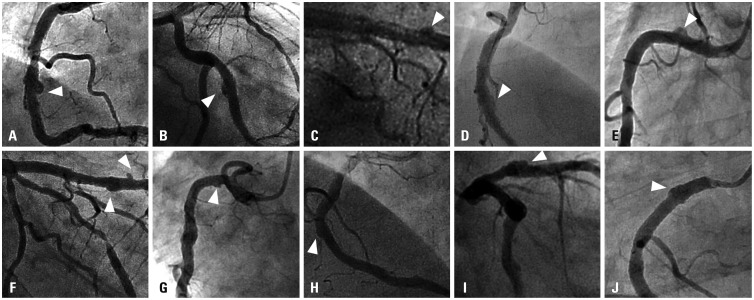
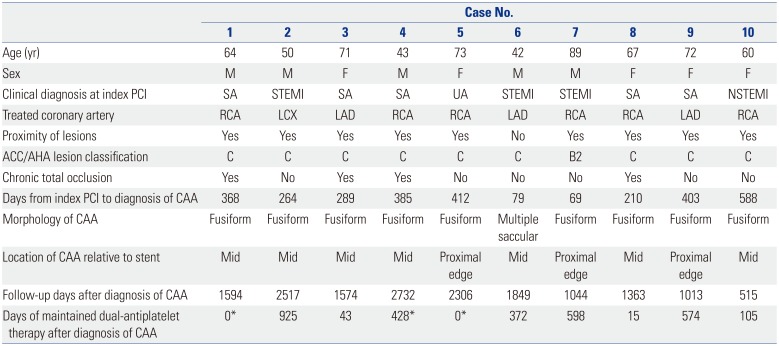
Among 976 consecutive patients (1245 lesions) who underwent follow-up angiography, a total of 10 CAAs (0.80% per lesion) in 10 patients (1.02% per patient) were detected at follow-up after second-generation DES implantation with a median implanted time of 329 days (interquartile range: 210–403 days). The individual profiles and angiographic morphologies of these patients with CAA are shown in
Table 1 and
Fig. 2. The incidence of CAA after second-generation DES implantation was similar to that of CAA after first-generation DES implantation according to our previous registry data (0.77% per lesion and 0.80% per patient).
4
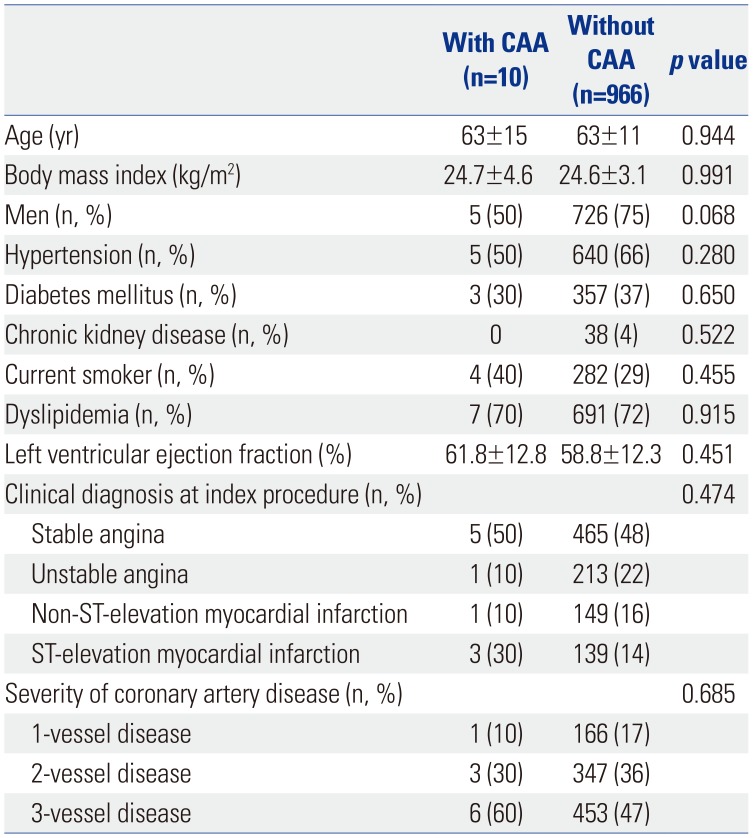
Clinical characteristics between the patients with CAA (n=10) and those without CAA (n=966) after second-generation DES placement are presented in
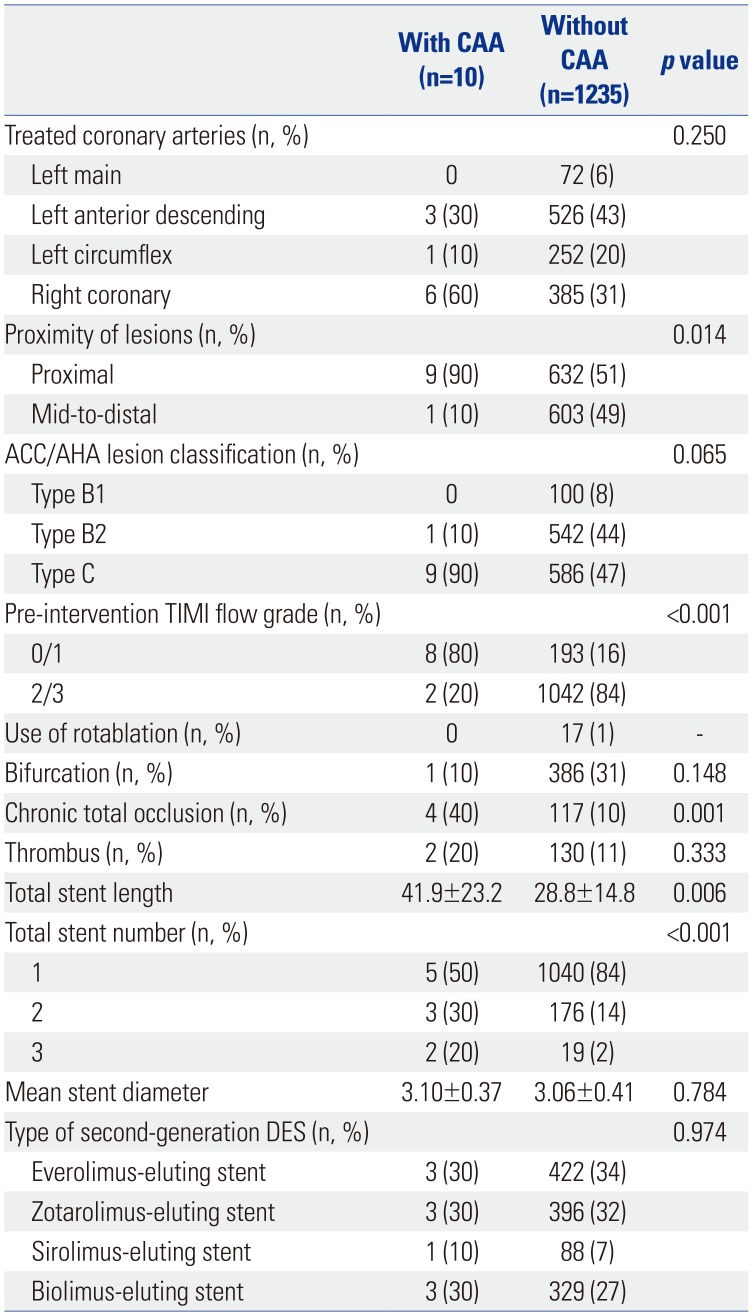
Table 2. There were no statistically significant differences in clinical characteristics between the two groups. Angiographic and procedural characteristics between the lesions with and without CAA after second-generation DES implantation are also presented in
Table 3. In comparison with the lesions without CAA, those with CAA had more proximal lesions than mid-to-distal lesions (90% vs. 51%,
p=0.014), a higher proportion of pre-intervention Thrombolysis in Myocardial Infarction (TIMI) scores of 0 or 1 flow (80% vs. 16%,
p<0.001), and more chronic total occlusions (40% vs. 10%,
p<0.001). Also, total stent length was significantly longer in the lesions with CAA versus those without CAA (41.9±23.2 mm vs. 28.8±14.8 mm,
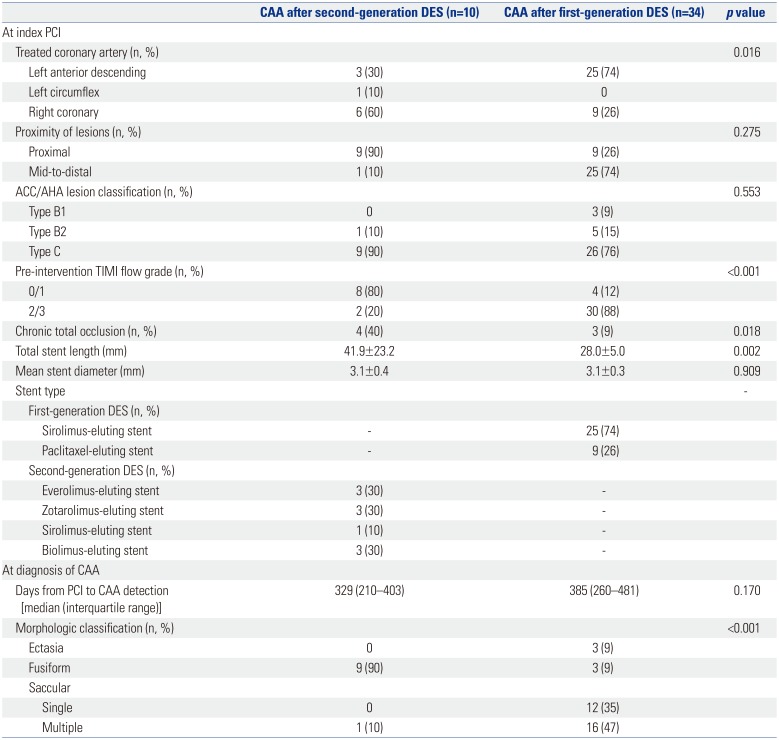
p=0.006). When compared to the lesions with CAA after first-generation DES implantation (n=34) (
Table 4), those with CAA after second-generation DES implantation had a significantly higher proportion of pre-intervention TIMI scores of 0 or 1 flow (80% vs. 12%,
p<0.001), more chronic total occlusions (40% vs. 9%,
p=0.018), and longer total stent length (41.9±23.2 mm vs. 28.0±5.0 mm,
p=0.002).
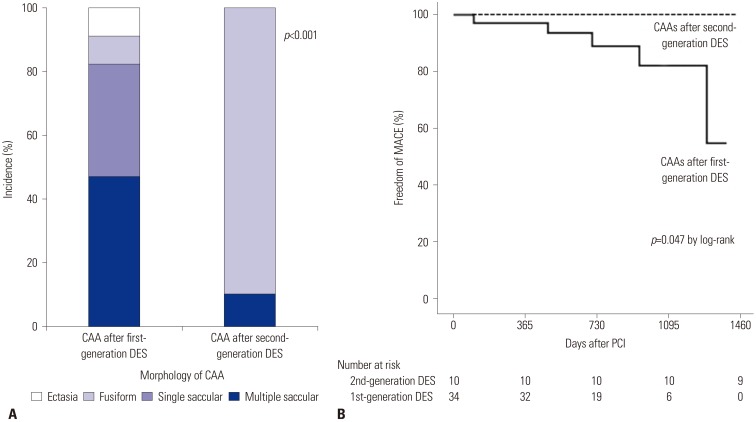
As for CAA morphology, the predominant form of CAA after second-generation DES implantation was significantly different from that of CAA after first-generation DES implantation (
p<0.001) (
Table 4,
Fig. 3A). CAAs after second-generation DES placement were predominantly of the single fusiform type (90%), whereas CAAs after first-generation DES placement were multiple saccular (47%) and single saccular (35%) types.
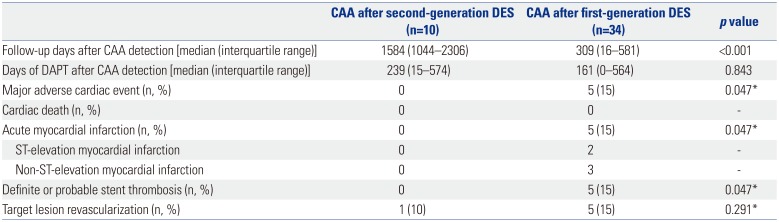
The clinical outcomes of CAA according to DES generation are presented in
Table 5 and
Fig. 3B. During a median follow-up of 309 days (interquartile range: 16–581 days) after CAA detection following first-generation DES implantation, a total of five cases with CAA (15%) experienced MACE.
4 These five adverse events were related to acute myocardial infarction with definite or probable stent thrombosis.
4 However, during a median follow-up of 1584 days (interquartile range: 1044–2306 days), no patients with CAA had MACE, and one patient with CAA received target lesion revascularization at the time of CAA detection (case 4 in
Table 1,
Supplementary Fig. 1, only online). Thus, CAAs after first-generation DES implantation had significantly higher MACE rates than did those after second-generation DES implantation (
p value by log-rank=0.047). The duration of dual-antiplatelet therapy after CAA detection was not different between CAAs after second-generation DES implantation versus those after first-generation DES implantation (
p=0.847).
DISCUSSION
The major findings of our study are that (1) CAAs after second-generation DES implantation were detected at incidence rates of 0.80% per lesion and 1.02% per patient, which were higher than values associated with CAA after first-generation DES implantation; (2) after implantation of second-generation DESs, CAAs were more frequently detected in correlation with the treatment of more complex lesions (e.g., pre-intervention TIMI flow grade 0 or 1, chronic total occlusions, and longer implanted stents); and (3) CAAs after second-generation DES implantation had different morphologies and might have more favorable clinical outcomes, compared with those after first-generation DES implantation.
Although the exact mechanisms remain unclear, several causes of CAAs have been suggested to date. The mechanical risk factors for CAAs after coronary intervention include arterial wall injury as well as dissection or rupture induced by high-pressure balloon inflation or oversized stent. Atherectomy and laser angioplasty have additionally been associated with the development of CAAs.
1011 Therefore, our findings of the predictors of CAAs after second-generation DES implantation, specifically more complex lesions, such as chronic total occlusions or those requiring longer stents, may be line with the suggested mechanisms. Similar to these findings, CAA development after first-generation DES placement was related to more severe complex lesions. Our previous registry data also indicated that the CAA development after first-generation DES placement occurs exclusively in complex (type B2/C) de novo lesions and that lesion length is significantly greater in patients with CAA than in those without CAA.
4 Interestingly, when we compared the observed CAAs present after second-generation DES implantation and those present after first-generation DES implantation, the CAAs after second-generation DES placement also had more complex lesions than did those after first-generation DES placement.
Other factors for the development of CAAs have also been proposed due to DES implantation.
1234 DESs have been used for reducing restenosis by administration of cytotoxic anti-restenosis drugs to inhibit endothelial cell and smooth muscle cell proliferation, and these antiproliferative effects have been suggested to increase the risk of CAAs because of the mechanism of delayed neointimal healing and reendotheliazation.
1213 Separately, a local hypersensitivity reaction to the polymer of the drug in the DESs may contribute to CCAs. The inflammatory response to the drugs increased eosinophilic or heterophilic infiltration into the vessel, as well as the occurrence of local toxic effects. These mechanisms induce weakening and disruption of the arterial wall, provoking arterial wall expansion and aneurysmal change.
131415 Although concerns about the development of CAAs after DES placement have decreased with greater use of second-generation DESs, these limitations still can be related to CAA onset even in the context of second-generation DES implantation. In our study, we documented incidence rates of CAAs after second-generation DES implantation of 0.80% per lesion and 1.02% per patient. According to previous reports on first-generation DESs, CAAs were detected with an incidence of 0.8% to 1.3%.
1234 Such numbers are relatively comparable to those regarding CAAs after second-generation DES use. However, importantly, different than the CAAs after first-generation implantation, the CAAs after second-generation implantation had different predominant morphologies and more favorable outcomes, reflecting the advances in DES technology. According to recent studies involving first-generation DESs, CAAs are frequently associated with adverse clinical events as a result of DES thrombosis and restenosis.
1234 In one investigation, Alfonso, et al.
1 found that, of 1197 patents with first-generation DESs, CAAs developed in 15 patients (1.25%) and the 1-year adverse event rate was 51%. Joo, et al.
3 followed up 78 patients with CAA after first-generation DES implantation and determined that the patients with CAAs displayed a significantly higher incidence of MACE than did those without CAAs, driven by target-lesion revascularization and myocardial infarction. Lastly, from our CAA registry of first-generation DESs, myocardial infarction with stent thrombosis occurred in 17.2% of the cases with CAA with 75% being on aspirin alone without clopidogrel.
4 However, notably, all three of these studies were conducted in patients treated with first-generation DES placement.
Our current study has some limitations. First, although we enrolled consecutive patients with follow-up angiography in our study, the percentages and indications were not pre-specified and differed from the cohorts with CAAs after first-generation DES placement. Also, selection bias is inevitable because all patients did not undergo follow-up angiography in our study. Second, a relatively small number of patients was analyzed, necessitating a larger group of initial follow-up patients to be studied for further confirmation. Also, the follow-up duration of the CAA after first-generation DES was shorter than that of the CAA after second-generation DES, because our previous CAA registry of the first-generation DES was not extended for longer follow up. Third, our patients did not undergo invasive intracoronary imaging studies, though these studies would be helpful to observe the development of late-acquired stent malapposition due to CAA.
In conclusion, CAAs after second-generation DES implantation were detected rarely at a similar incidence to that of CAAs after first-generation DES implantation. However, patients with second-generation DESs might demonstrate more favorable clinical outcomes, compared to those after first-generation DES implantation.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download