Abstract
Purpose
We aimed to analyze the frequency of eosinophilia-associated diseases and to search for possible markers that may be useful for their differential diagnosis.
Methods
We retrospectively reviewed the medical records of 148 patients with peripheral blood eosinophil count of more than 500/μL who visited the Allergy Department of Chonnam National University Hospital for the first time from January to December 2016. Blood eosinophilia was categorized as mild (<1,500/μL), moderate (1,500–5,000/μL), and severe (>5,000/μL).
Results
Blood eosinophilia was mostly caused by allergic diseases (41.9%), parasitic infestation (23.6%), and drug allergy (19.6%). Eosinophil count was higher in patients with parasitic infestation (P<0.01), drug allergy (P<0.01), hypereosinophilic syndrome (HES, P<0.001), or eosinophilic granulomatosis with polyangiitis (EGPA, P<0.001) than in those with allergic diseases. The eosinophilic cationic protein level was higher in patients with HES than in those with allergic diseases (P<0.05) and parasitic infestation (P<0.05). The total IgE level was lower in patients with HES than in those with parasitic infestation (P<0.05) and EGPA (P<0.05). The vitamin B12 level was higher in patients with HES than in those with parasitic infestation (P<0.05). There was no statistically significant difference in tryptase levels between the groups. The most common cause of mild eosinophilia was allergic diseases (59.8%), followed by parasitic infestation (22.7%) and drug allergy (13.4%). The common causes of moderate eosinophilia were drug allergy (37.8%), parasitic infestation (29.7%), and allergic diseases (10.8%). The common causes of severe eosinophilia were EGPA (28.6%), HES (21.4%), parasitic infestation (14.3%), and drug allergy (14.3%).
Conclusion
Common causes of blood eosinophilia in patients who visit the allergy department are allergic diseases, parasitic infestation, and drug allergy. Several markers, including eosinophil count, total IgE, and vitamin B12, may be useful for the differential diagnosis of eosinophilia-associated diseases.
Go to : 
References
1. Roufosse F, Weller PF. Practical approach to the patient with hypereosinophilia. J Allergy Clin Immunol. 2010; 126:39–44.

2. Kita H. Eosinophils: multifaceted biological properties and roles in health and disease. Immunol Rev. 2011; 242:161–77.

3. Kobayashi H. Effect of c-kit ligand (stem cell factor) in combination with interleukin-5, granulocyte-macrophage colony-stimulating factor, and interleukin-3, on eosinophil lineage. Int J Hematol. 1993; 58:21–6.
5. Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014; 289:17406–15.

7. Bullock ED, Johnson EM Jr. Nerve growth factor induces the expression of certain cytokine genes and bcl-2 in mast cells. Potential role in survival promotion. J Biol Chem. 1996; 271:27500–8.
8. Brigden M, Graydon C. Eosinophilia detected by automated blood cell counting in ambulatory North American outpatients. Incidence and clinical significance. Arch Pathol Lab Med. 1997; 121:963–7.
9. Magnaval JF, Laurent G, Gaudré N, Fillaux J, Berry A. A diagnostic protocol designed for determining allergic causes in patients with blood eosinophilia. Mil Med Res. 2017; 4:15.

10. Lowe D, Jorizzo J, Hutt MS. Tumour-associated eosinophilia: a review. J Clin Pathol. 1981; 34:1343–8.

11. Jin JJ, Butterfield JH, Weiler CR. Hematologic malignancies identified in patients with hypereosinophilia and hypereosinophilic syndromes. J Allergy Clin Immunol Pract. 2015; 3:920–5.

12. Williams KW, Milner JD, Freeman AF. Eosinophilia associated with disorders of immune deficiency or immune dysregulation. Immunol Allergy Clin North Am. 2015; 35:523–44.

13. Skiest DJ, Keiser P. Clinical significance of eosinophilia in HIV-infected individuals. Am J Med. 1997; 102:449–53.

15. Tefferi A, Patnaik MM, Pardanani A. Eosinophilia: secondary, clonal and idiopathic. Br J Haematol. 2006; 133:468–92.

16. Webb TA, Li CY, Yam LT. Systemic mast cell disease: a clinical and hema-topathologic study of 26 cases. Cancer. 1982; 49:927–38.

17. Chung J, Nam D, Lee S, Lee E, Hahn J, Ko Y. A clinical study on eosinophilia: with a report of 5 cases of hypereosinophilic syndrome. Korean J Hematol. 1988; 23:127–37.
18. Shin K, Choi Y, Chae S, Hyung S. A clinical study of cause of Eosinophilia. Chungbuk Med J. 1996; 6:105–14.
19. Kim DW, Shin MG, Yun HK, Kim SH, Shin JH, Suh SP, et al. Incidence and causes of hypereosinophilia in the patients of a university hospital. Korean J Lab Med. 2009; 29:185–93.
20. Cacoub P, Musette P, Descamps V, Meyer O, Speirs C, Finzi L, et al. The DRESS syndrome: a literature review. Am J Med. 2011; 124:588–97.

21. Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy. 2013; 43:850–73.

23. Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 1990; 33:1094–100.

24. Prussin C. Eosinophilic gastroenteritis and related eosinophilic disorders. Gastroenterol Clin North Am. 2014; 43:317–27.

25. Simon HU, Rothenberg ME, Bochner BS, Weller PF, Wardlaw AJ, Wechsler ME, et al. Refining the definition of hypereosinophilic syndrome. J Allergy Clin Immunol. 2010; 126:45–9.

27. Sheikh J, Weller PF. Clinical overview of hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007; 27:333–55.

28. Yoon SY, Baek S, Park SY, Shin B, Kwon HS, Cho YS, et al. Clinical course and treatment outcomes of toxocariasis-related eosinophilic disorder. Medicine (Baltimore). 2018; 97:e12361.

29. Curtis C, Ogbogu PU. Evaluation and differential diagnosis of persistent marked eosinophilia. Immunol Allergy Clin North Am. 2015; 35:387–402.

30. Kim KM, Bae MH, Kim YM, Cho MJ, Kwak MJ, Kim SH, et al. Cause and incidence of eosinophilia in children: a single center study in one year. Allergy Asthma Respir Dis. 2014; 2:358–61.

32. Chen YY, Khoury P, Ware JM, Holland-Thomas NC, Stoddard JL, Gur-prasad S, et al. Marked and persistent eosinophilia in the absence of clinical manifestations. J Allergy Clin Immunol. 2014; 133:1195–202.

33. Adkinson NF, Middleton E. Middleton's allergy: principles and practice. 8th ed.Philadelphia (PA): Elsevier/Saunders;2014.
34. Wu EY, Hernandez ML, Jennette JC, Falk RJ. Eosinophilic granulomatosis with polyangiitis: clinical pathology conference and review. J Allergy Clin Immunol Pract. 2018; 6:1496–504.

35. Rudzińska M, Kowalewska B, Sikorska K. Clinical usefulness of Western blotting and ELISA avidity for the diagnosis of human toxocariasis. Para-site Immunol. 2017; 39.

36. Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010; 125(2 Suppl 2):S73–80.

37. Bonnin AJ, Montealegre F, Gewurz A. Association of cigarette smoking with elevated serum IgE levels in Hispanic Puerto Rican men. Ann Allergy. 1991; 67:609–11.
38. Arendt JF, Nexo E. Unexpected high plasma cobalamin: proposal for a diagnostic strategy. Clin Chem Lab Med. 2013; 51:489–96.
39. Bochner BS. The eosinophil: For better or worse, in sickness and in health. Ann Allergy Asthma Immunol. 2018; 121:150–5.
Go to : 
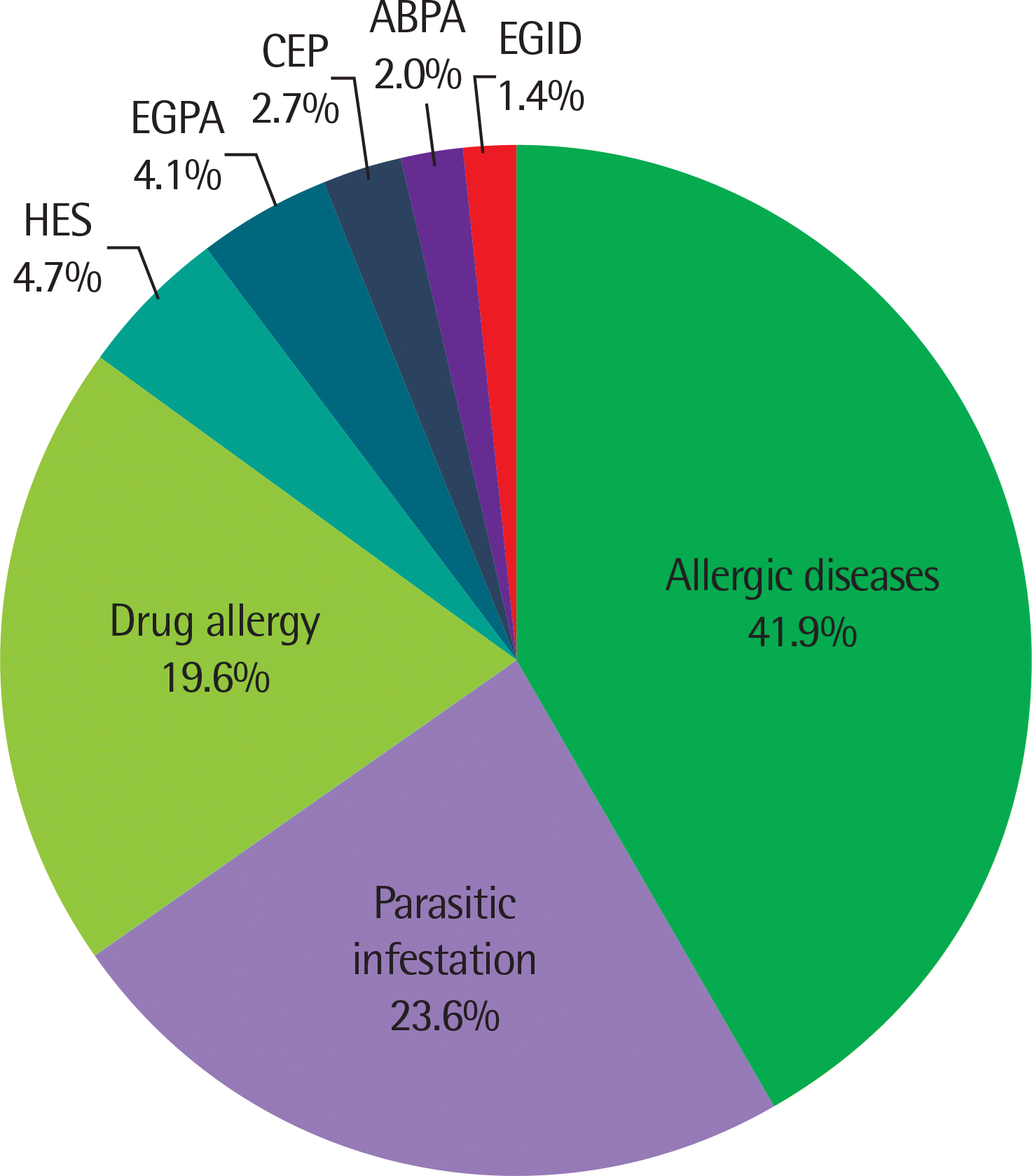 | Fig. 1.Frequency of eosinophilia-associated diseases in all patients (n=148). ABPA, allergic bronchopulmonary aspergillosis; CEP, chronic eosinophilic pneumonia; EGID, eosinophilic gastrointestinal disorder; EGPA, eosinophilic granulomatosis with polyangiitis; HES, hypereosinophilic syndrome. |
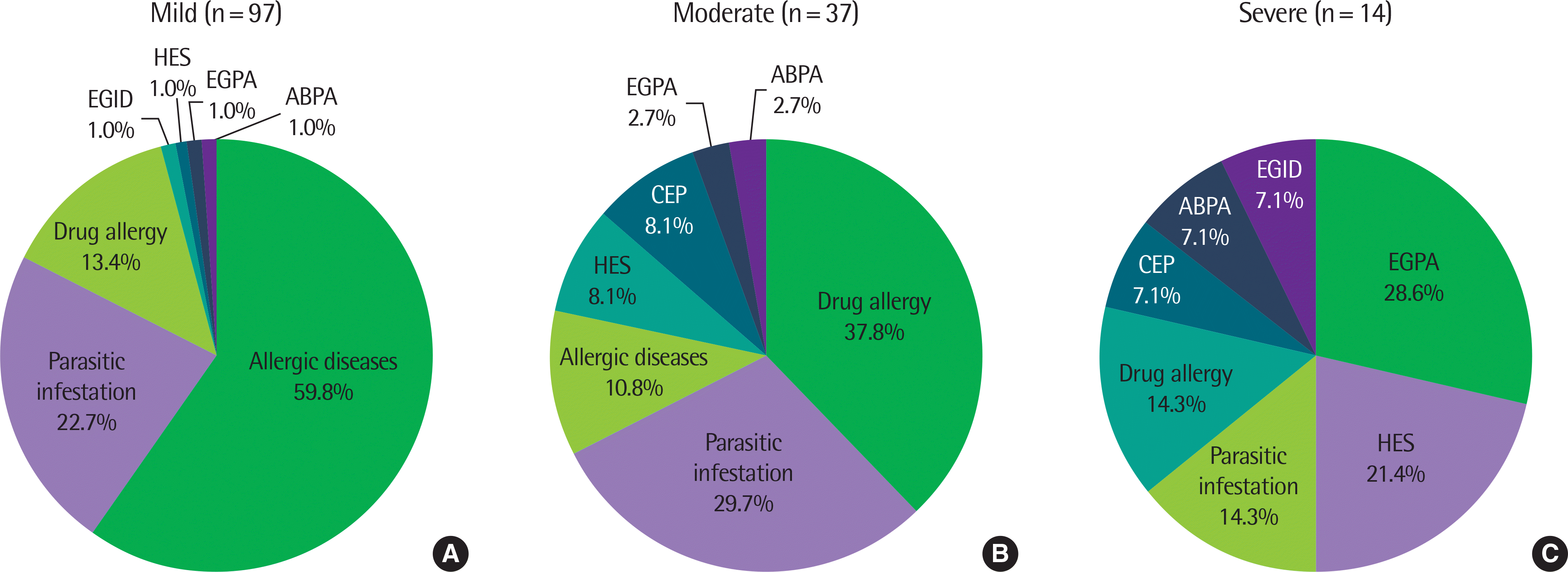 | Fig. 2.Frequency of eosinophilia-associated diseases in patients with mild (A), moderate (B), and severe (C) eosinophilia. ABPA, allergic bronchopulmonary aspergillosis; CEP, chronic eosinophilic pneumonia; EGID, eosinophilic gastrointestinal disorder; EGPA, eosinophilic granulomatosis with polyangiitis; HES, hypereosinophilic syndrome. |
Table 1.
Comparison of characteristics among the diverse eosinophilia-associated diseases
| Variable | Allergic diseases (n=62) | Parasitic infestation (n=35) | Drug allergy (n=29) | HES (n=7) | EGPA (n=6) | CEP (n=4) | ABPA (n=3) | EGID (n=2) | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | 52 (16–79) | 59 (18–78) | 55 (18–86) | 48 (21–76) | 78 (73–86)*, † | 50 (46–67) | 53 (42–58) | 80 (76–84) | 0.001 |
| Female sex | 35 (56.5)‡,§ | 6 (17.1) | 7 (24.1) | 4 (57.1)‡ | 4 (66.7)‡,§ | 4 (100)‡,§ | 2 (66.7)‡ | 2 (100)‡, § | <0.001 |
| Blood eosinophil (%) | 10.1(5.2–46.4) | 17.4*(7.2–86.7) | 15.7* (5.5–48.7) | 45.2*(7.2–86.7) (14.4–65.5) | 47.7*(7.2–86.7) (9.8–71.4) | 23.5 (13.7–32.1) | 16.6 (13.8–43.0) | 27.1 (10.4–43.7) | <0.001 |
| Blood eosinophil (/μL) | 700 μ (500–3,400) | 1,100*(7.2–86.7) (500–39,400) | 1,600*(7.2–86.7) (500–12,250) | 5,000*(7.2–86.7) (1,400–12,200) | 8,385*(7.2–86.7) (1,300–41,450) | 2,200*(7.2–86.7) (1,600–5,430) | 1,600 (1,300–5,010) | 3,095 (640–5,550) | <0.001 |
| ECP | 36.6 | 28.0 | 49.9 | 191.5*(7.2–86.7), ‡ | 154.5 | 117.0 | 120.0 | 88.6 | NA |
| (μg/L) | (0–153) (n=44) | (3.9–200) (n=23) | (26.6–137) (n=7) | (53.3–200) (n=6) | (108–200) (n=4) | (12.4–200) (n=3) | (5.6–180) (n=3) | (n=1) | |
| Total IgE | 320 | 700 | 93 | 71‡, ∥ | 7,970 | 570 | 1,050 | 590 | 0.003 |
| (IU/mL) | (0–11,700) (n=58) | (0–11,800) (n=34) | (0–41,600) (n=18) | (0–697) (n=7) | (181–23,500) (n=6) | (118–2,050) (n=4) | (746–1,630) (n=3) | (186–994) (n=2) | |
| Vitamin B12 (pmol/L) | 690 (253–1,306) | 442 (183–803) | 579 (395–1,476) | 765‡(413–1,476) | 790 (402–1,035) | 714 (n=1) | ND | 1,217 (n=1) | NA |
| (n=7) | (n=32) | (n=8) | (n=7) | (n=5) | |||||
| Tryptase (μg/L) | 4.21 (1.35–5.99) (n=9) | 3.24 (1.58–7.31) (n=32) | 3.81 (0–10.4) (n=9) | 4.39 (0–19.4) (n=7) | 3.35 (0–14.8) (n=5) | 3.09 (1.83–4.35) (n=2) | ND | 4.79 (n=1) | NA |
Values are presented as median (range) or number (%). ABPA, allergic bronchopulmonary aspergillosis; CEP, chronic eosinophilic pneumonia; ECP, eosinophilic cationic protein; EGID, eosinophilic gastrointestinal disorder; EGPA, eosinophilic granulomatosis with polyangiitis; HES, hypereosinophilic syndrome; NA, not available; ND, no data.
Table 2.
Comparison of characteristics among the diverse allergic diseases
Table 3.
Comparison of characteristics among the diverse parasitic infestations
| Variable | Toxocariasis (n=26) | Clonorchiasis (n=2) | Paragonimiasis (n=2) | P-value |
|---|---|---|---|---|
| Age (yr) | 61 (30–77) | 61 (50–71) | 24 (18–29)* | 0.066 |
| Female sex | 2 (7.7) | 0 (0) | 1 (50) | 0.140 |
| Blood eosinophil (%) | 14.9 (10.7–37.0) | 12.5 (7.4–17.5) | 55.9 (25.0–86.7)* | 0.081 |
| Blood eosinophil (/μL) | 1,050 (700–7,180) | 800 (500–1,100) | 20,730 (2,060–39,400) | 0.119 |
| ECP (μg/L) | 25.7 (9.4–200) (n=16) | 35.9 (26.4–45.3) (n=2) | 132.1 (64.2–200) (n=2) | 0.248 |
| Total IgE (IU/mL) | 887 (0–11,800) | 251 (120–382) | 1,113 (715–1,510) | 0.302 |
| (n=26) | (n=2) | (n=2) |
Table 4.
Comparison of characteristics among the diverse drug allergies
| Variable | Drug rash (n=9) | Drug fever (n=6) | DRESS syndrome (n=9) | Exfoliative dermatitis (n=3) | P-value |
|---|---|---|---|---|---|
| Age (yr) | 51 (18–83) | 72 (29–82) | 47 (24–65) | 83 (77–86)* | 0.029 |
| Female sex | 2 (22.2) | 1 (16.7) | 3 (33.3) | 1 (33.3) | 0.879 |
| Blood eosinophil (%) | 11.2 (5.5–22.6) | 12.8 (6.9–17.9) | 24.9 (8.8–48.7) | 17.6 (16.2–37.0) | 0.033 |
| Blood eosinophil (/μL) | 800 (500–2,650) | 940 (500–2,000) | 2,700 (700–12,250) | 1,600 (900–4,500) | 0.029 |
| ECP (μg/L) | 73.0 (37.9–108) (n=2) | ND | 46.1 (26.6–79.0) (n=4) | ND | NA |
| Total IgE (IU/mL) | 114 (39–2,990) (n=3) | 58 (17–91) (n=4) | 82 (0–3,140)(n=8) | 108 (n=1) | NA |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download