Abstract
Purpose
Recently, the incidence of refractory Mycoplasma pneumoniae (MP) pneumonia has increased in Korea. Given that its early diagnosis is helpful in selection of the treatment, this study aimed at investigating the value of the antimycoplasma antibody (IgM) for early diagnosis of MP pneumonia.
Methods
A total of 315 children admitted with MP pneumonia from September 2015 to May 2016 were investigated with the IgM and polymerase chain reaction (PCR) for the diagnosis of MP pneumonia. Specifically, patients were grouped into nonrefractory respiratory MP and refractory MP groups according to their response to macrolide therapy.
Results
In the 44 PCR-negative seroconversed children, seroconversed IgM was more frequent in the refractory MP group compared with the nonrefractory respiratory MP group with statistical significance (P<0.001). In the 264 IgM-positive children, the time of antibody reaction was more delayed in the refractory MP group compared to the nonrefractory respiratory MP group with statistical significance (P<0.001).
Conclusion
This study showed that there was a higher incidence of seroconversed IgM and delayed antibody reaction in the refractory MP group. In children with suspect MP pneumonia, follow-up studies of antibody are necessary, even through initial antibody and PCR showed negative findings. In addition, this result may suggest that the diagnosis of refractory MP pneunomia will be helpful in establishing the strategy of the treatment.
Go to : 
References
1. Youn YS, Lee KY, Hwang JY, Yim JW, Kang JH, Lee JS. Comparison of diagnostic methods and the changes of IgG subclasses in children with Mycoplasma pneumoniae pneumonia. Pediatr Allergy Respir Dis. 2009; 19:137–45.
2. Seo BO, Kang JW, Kim BG, Lee EY, Kim SW. Usefulness of short-term follow-up enzyme immunoassay in Mycoplasma pneumoniae Infection. Pediatr Allergy Respir Dis. 2008; 18:305–15.
3. Shin JE, Cheon BR, Shim JW, Kim DS, Jung HL, Park MS, et al. Increased risk of refractory Mycoplasma pneumoniae pneumonia in children with atopic sensitization and asthma. Korean J Pediatr. 2014; 57:271–7.
4. Hong KB, Choi EH, Lee HJ, Lee SY, Cho EY, Choi JH, et al. Macrolide resistance of Mycoplasma pneumoniae, South Korea, 2000–2011. Emerg Infect Dis. 2013; 19:1281–4.
5. Yang HJ, Song DJ, Shim JY. Mechanism of resistance acquisition and treatment of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Korean J Pediatr. 2017; 60:167–74.

6. Chung EH. Serological diagnosis of Mycoplasma pneumoniae infections. Korean J Pediatr Infect Dis. 2006; 13:21–30.

7. Kim S, Um TH, Cho CR. Evaluation of the chorus Mycoplasma pneumoniae IgM assay for the serological diagnosis of Mycoplasma pneumoniae infection. J Lab Med Qual Assur. 2012; 34:57–62.
8. You SY, Jwa HJ, Yang EA, Kil HR, Lee JH. Effects of methylprednisolone pulse therapy on refractory Mycoplasma pneumoniae pneumonia in children. Allergy Asthma Immunol Res. 2014; 6:22–6.
9. Bernet C, Garret M, de Barbeyrac B, Bebear C, Bonnet J. Detection of Mycoplasma pneumonia by using the polymerase chain reaction. J Clin Microbiol. 1989; 27:2492–6.
10. Tully JG, Rose DL, Whitcomb RF, Wenzel RP. Enhanced isolation of Mycoplasma pneumoniae from throat washings with a newly-modified culture medium. J Infect Dis. 1979; 139:478–82.
11. Barker CE, Sillis M, Wreghitt TG. Evaluation of Serodia Myco II particle agglutination test for detecting Mycoplasma pneumoniae antibody: comparison with mu-capture ELISA and indirect immunofluorescence. J Clin Pathol. 1990; 43:163–5.

12. Waris ME, Toikka P, Saarinen T, Nikkari S, Meurman O, Vainionpää R, et al. Diagnosis of Mycoplasma pneumoniae pneumonia in children. J Clin Microbiol. 1998; 36:3155–9.
13. Aubert G, Pozzetto B, Gaudin OG, Hafid J, Mbida AD, Ros A. Evaluation of five commercial tests: complement fixation, microparticle agglutination, indirect immunofluorescence, enzyme-linked immunosorbent assay and latex agglutination, in comparison to immunoblotting for Mycoplasma pneumoniae serology. Ann Biol Clin (Paris). 1992; 50:593–7.
14. Chia WK, Spence L, Dunkley L, Bradbury W. Development of urease conjugated enzyme-linked immunosorbent assays (ELISA) for the detection of IgM and IgG antibodies against Mycoplasma pneumoniae in human sera. Diagn Microbiol Infect Dis. 1988; 11:101–7.

15. Shin YH, Lee BC, Song TW, Kim KW, Lee KE, Kim ES, et al. Diagnostic availability of PCR and ELISA in Mycoplasma pneumoniae pneumonia. Pediatr Allergy Respir Dis. 2006; 16:47–56.
16. Beersma MF, Dirven K, van Dam AP, Templeton KE, Claas EC, Goossens H. Evaluation of 12 commercial tests and the complement fixation test for Mycoplasma pneumoniae-specific immunoglobulin G (IgG) and IgM antibodies, with PCR used as the “gold standard”. J Clin Microbiol. 2005; 43:2277–85.
17. Thacker WL, Talkington DF. Comparison of two rapid commercial tests with complement fixation for serologic diagnosis of Mycoplasma pneumoniae infections. J Clin Microbiol. 1995; 33:1212–4.

18. Kim HJ, Nam SY, Yoon HS, Kim WK. Correlation between the diagnostic validity of enzymeimmunoassay and the hyperreactivity of the respiratory tract for the diagnosis of Mycoplasma pneumoniae. Pediatr Allergy Respir Dis. 2007; 17:394–403.
19. Principi N, Esposito S. Emerging role of Mycoplasma pneumoniae and Chlamydia pneumoniae in paediatric respiratory-tract infections. Lancet Infect Dis. 2001; 1:334–44.

20. van Kuppeveld FJ, Johansson KE, Galama JM, Kissing J, Bölske G, Hjelm E, et al. 16S rRNA based polymerase chain reaction compared with culture and serological methods for diagnosis of Mycoplasma pneumoniae infection. Eur J Clin Microbiol Infect Dis. 1994; 13:401–5.
21. Skakni L, Sardet A, Just J, Landman-Parker J, Costil J, Moniot-Ville N, et al. Detection of Mycoplasma pneumoniae in clinical samples from pediatric patients by polymerase chain reaction. J Clin Microbiol. 1992; 30:2638–43.

22. Williamson J, Marmion BP, Worswick DA, Kok TW, Tannock G, Herd R, et al. Laboratory diagnosis of Mycoplasma pneumoniae infection. 4. Antigen capture and PCR-gene amplification for detection of the Mycoplasma: problems of clinical correlation. Epidemiol Infect. 1992; 109:519–37.
23. Ieven M, Ursi D, Van Bever H, Quint W, Niesters HG, Goossens H. Detection of Mycoplasma pneumoniae by two polymerase chain reactions and role of M. pneumoniae in acute respiratory tract infections in pediatric patients. J Infect Dis. 1996; 173:1445–52.

24. Nadal D, Bossart W, Zucol F, Steiner F, Berger C, Lips U, et al. Communi-ty-acquired pneumonia in children due to Mycoplasma pneumoniae: diagnostic performance of a seminested 16S rDNA-PCR. Diagn Microbiol Infect Dis. 2001; 39:15–9.
25. Daxboeck F, Krause R, Wenisch C. Laboratory diagnosis of Mycoplasma pneumoniae infection. Clin Microbiol Infect. 2003; 9:263–73.

26. Morozumi M, Ito A, Murayama SY, Hasegawa K, Kobayashi R, Iwata S, et al. Assessment of realtime PCR for diagnosis of Mycoplasma pneumoniae pneumonia in pediatric patients. Can J Microbiol. 2006; 52:125–9.
27. Yoon KN, Park SW, Lee EH, Lee WB, Hwang KT, Lee HJ. Clinical utility of the sputum polymerase chain reaction obtained by nebulizer in the diagnosis of Mycoplasma pneumonia pneumonia. J Korean Pediatr Soc. 1999; 42:348–54.
28. Lee HJ, Kim ES, Jeong HJ, Rha YH, Chung SJ, Cha SH. Diagnostic availability of PCR in the Mycoplasma pneumonia pneumonia of children. Pediatr Allergy Respir Dis (Korea). 2005; 14:358–65.
29. Kim JH, Kim E, Kwon JH, Seo WH, Yoo Y, Choung JT, et al. Clinical characteristics of respiratory viral coinfection in pediatric Mycoplasma pneumoniae pneumonia. Allergy Asthma Respir Dis. 2017; 5:15–20.
30. An SH, Cho HJ, Baek HS, Sung MS, Yoon JW, Choi SH, et al. Clinical features of Mycoplasma pneumonia in comparison with viral pneumonia in children: a multicenter, cross-sectional study. Allergy Asthma Respir Dis. 2018; 6:155–60.
31. Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004; 17:697–728.
Go to : 
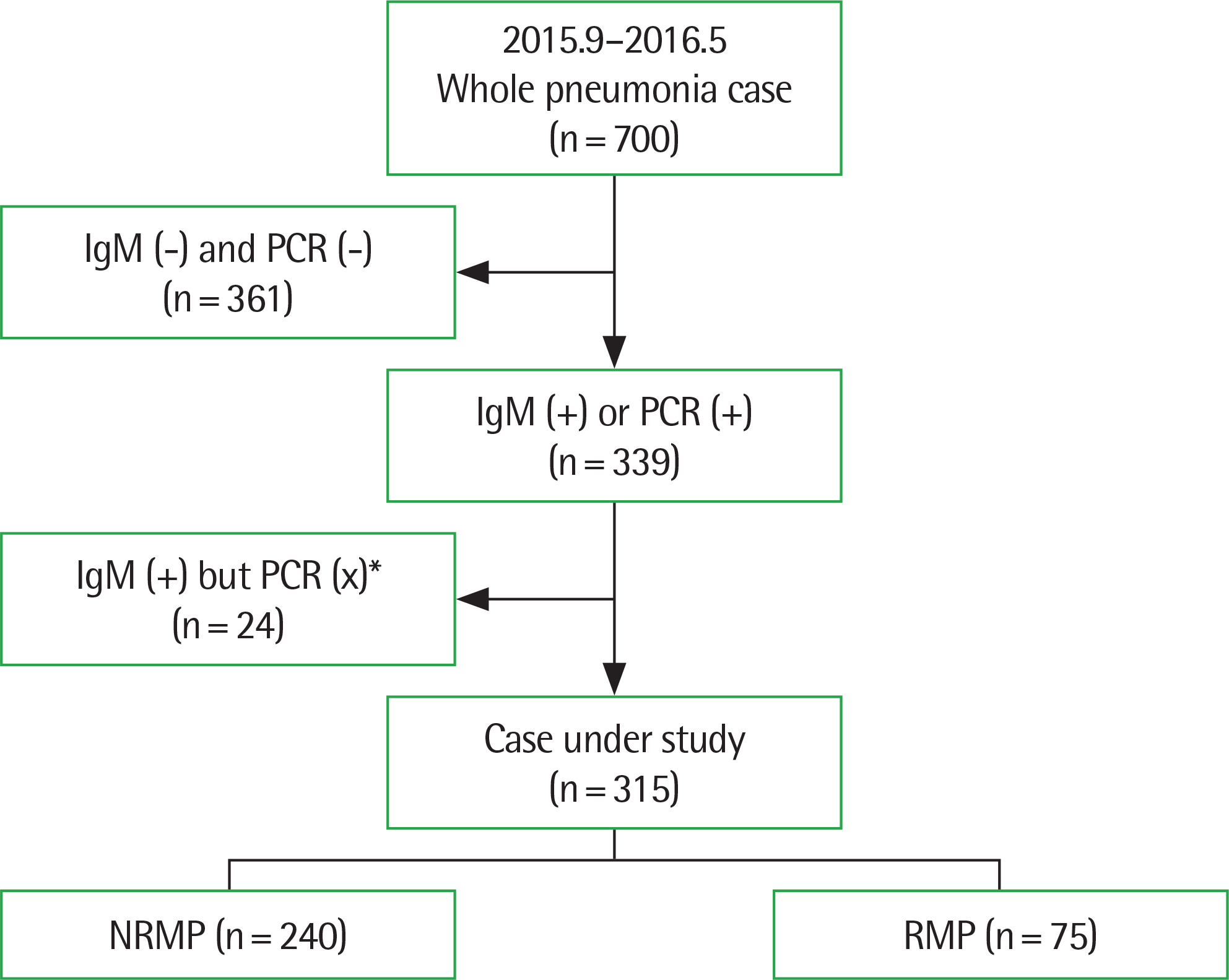 | Fig. 1.Patient flow chart. PCR, polymerase chain reaction; NRMP, nonrefractory Mycoplasma pneumoniae; RMP, refractory Mycoplasma pneumonia. *PCR(X): do not perform PCR test. |
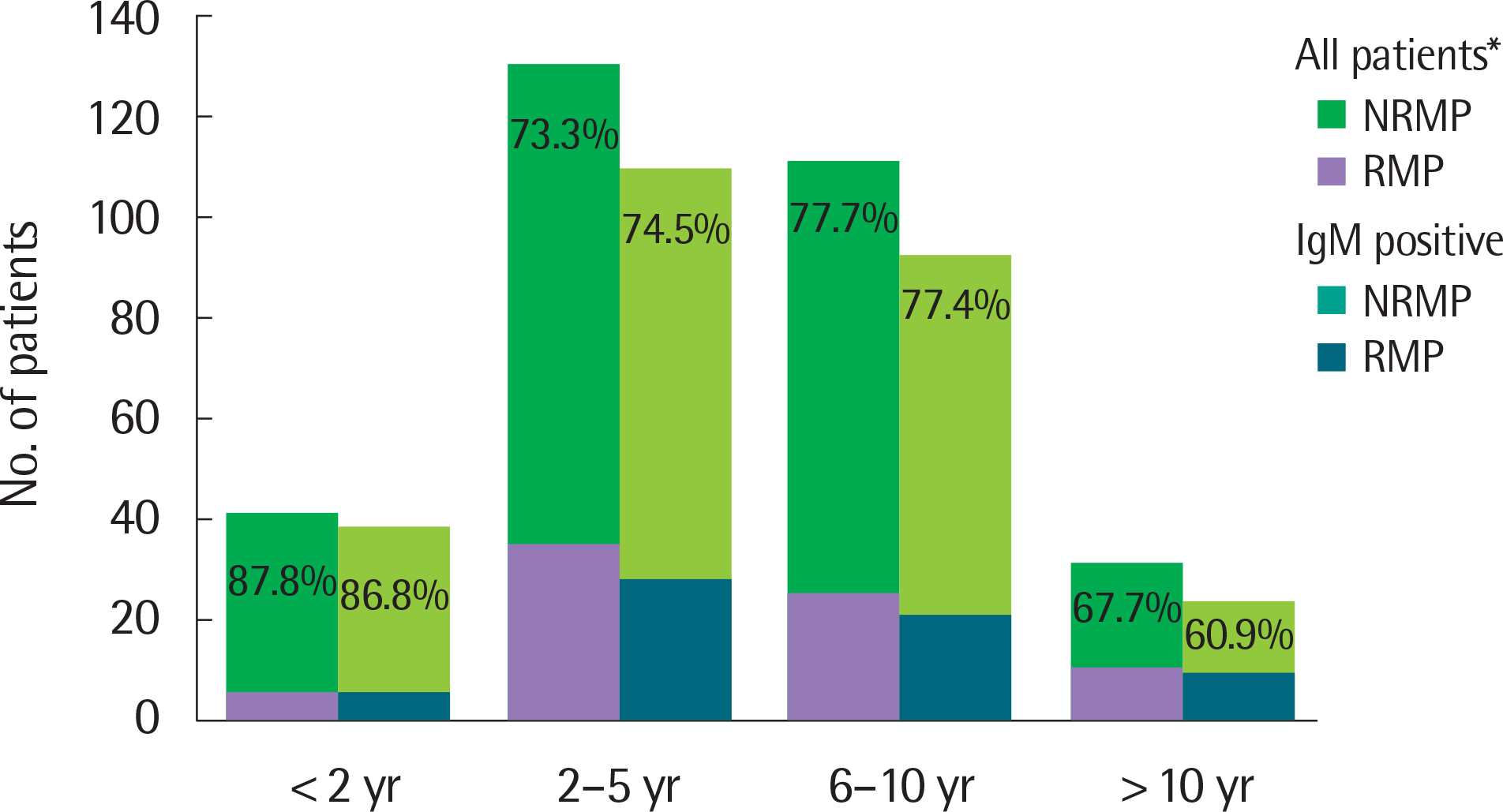 | Fig. 2.Age distribution. NRMP, nonrefractory Mycoplasma pneumoniae; RMP, refractory Mycoplasma pneumonia. †All patients included in this study. |
Table 1.
Demographic features of the subjects (n=315)
| Characteristic | Value |
|---|---|
| Sex, male:female | 151:164 (47.9:52.1) |
| Age (yr) | |
| Mean±SD | 6.08±3.84 |
| Range | 0.58–18 |
| <2 | 41 (13) |
| 2–5 | 131 (41.6) |
| 6–10 | 112 (35.6) |
| >10 | 31 (9.8) |
| IgM/PCR result | |
| +/+ | 100 (31.7) |
| +/- | 164 (52.1) |
| –/+ | 51 (16.2) |
| Seroconversed IgM (IgM/PCR initial result → next result) | 44 |
| –/+ → +/+* | 15 (34.1) |
| −/− → +/-† | 29 (65.9) |
Table 2.
Demographic features of the subjects with positive result (n=315)
| Variable | PCR (+)* | IgM (+)† | PCR (+) & IgM (+) |
|---|---|---|---|
| No. of subjects | 151 (47.9) | 264 (83.8) | 100 (31.7) |
| Sex, male:female | 75:76 (49.7:50.3) | 123:141 (46.6:53.4) | 47:53 (47:53) |
| Age (yr) | 6.77±3.96 | 5.88±3.77 | 6.62±5.82 |
| Duration of fever (day) | |||
| Before admission | 4.74±2.28 | 4.44±2.41 | 4.99±6.62 |
| After admission | 2.55±2.05 | 2.56±2.25 | 2.64±2.19 |
| Total duration | 7.30±3.10 | 7.00±3.29 | 7.65±3.42 |
| Positive rate | |||
| NRMP | 114 (47.5) | 201 (83.8) | 75 (31.3) |
| RMP | 37 (49.3) | 63 (84) | 25 (33.3) |
Table 3.
Demographic features of the subjects in NRMP and RMP groups (n=315)
Table 4.
IgM and PCR positive rate in NRMP and RMP groups
| Variable | NRMP | RMP | P-value |
|---|---|---|---|
| IgM/PCR result | 40 | 75 | |
| +/+ | 75 (31.3) | 25 (33.3) | 0.735 |
| +/- | 126 (52.5) | 38 (50.7) | 0.781 |
| –/+ | 39 (16.3) | 12 (16) | 0.959 |
| Seroconversed IgM* | 20 | 24 | |
| –/+ → +/+† | 9 (45) | 6 (25) | 0.131 |
| −/− → +/−‡ | 11 (55) | 18 (75) | 0.000 |
Table 5.
Evaluation time of antibody in IgM positive group (including serocon-ersed IgM group)
| Variable | NRMP (n=201) | RMP (n=63) | P-value |
|---|---|---|---|
| Admission (day)* | |||
| <5 | 199 (99) | 50 (79.4) | 0.985 |
| ≥5, <10 | 2 (1) | 12 (19) | 0.923 |
| ≥10 | 0 (0) | 1 (1.6) | |
| Mean±SD | 1.28±0.90 | 2.56±2.34 | 0.000 |
| Range | 0–14 | 1–14 | |
| Fever (day)† | |||
| <5 | 96 (47.8) | 13 (20.6) | 0.000 |
| ≥5, <10 | 98 (48.8) | 41 (65.1) | 0.024 |
| ≥10 | 7 (3.5) | 9 (14.3) | 0.002 |
| Mean±SD | 4.76±2.59 | 6.54±2.78 | 0.000 |
| Range | 0–14 | 1–14 |
Table 6.
Switching time of antibody in seroconversed IgM group
| Variable (days) | NRMP (n=20) | RMP† (n=24) | P-value |
|---|---|---|---|
| Admission (day)* | |||
| <5 | 18 (90) | 11 (45.8) | 0.002 |
| ≥5, <10 | 2 (10) | 12 (50) | 0.005 |
| ≥10 | 0 (0) | 1 (4.2) | 0.356 |
| Mean±SD | 3.80±1.06 | 5.08±2.00 | 0.010 |
| Range | 2–7 | 2–11 | |
| Fever (day)† | |||
| <5 | 0 (0) | 0 (0) | |
| ≥5, <10 | 17 (85) | 17 (70.8) | 0.264 |
| ≥10 | 3 (15) | 7 (29.2) | 0.264 |
| Mean±SD | 8.0±1.52 | 8.38±1.52 | 0.512 |
| Range | 5–11 | 5–13 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download