This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Hip fracture surgery (HFS) is often associated with perioperative blood loss, and it frequently necessitates transfusion. However, the hemoglobin (Hb) threshold for transfusion remains controversial in hip fracture patients. We evaluated the usefulness of the restrictive strategy and preoperative intravenous iron supplementation in HFS.
Methods
We retrospectively reviewed the medical records of 1,634 patients (> 60 years of age) who underwent HFS between May 2003 and June 2014 and were followed up for 1 year or more after surgery. We used the liberal transfusion strategy until May 2009 to determine the transfusion threshold; afterwards, we switched to the restrictive transfusion strategy. Patients with the restrictive transfusion strategy (restrictive group) received intravenous iron supplementation before surgery. We compared the transfusion rate, morbidity, and mortality of the restrictive group with those of the patients with the liberal transfusion strategy (liberal group).
Results
Preoperative intravenous iron supplementation was not associated with any adverse reactions. The transfusion rate was 65.3% (506/775) in the liberal group and 48.2% (414/859) in the restrictive group (p < 0.001). The mean hospital stay was shorter in the restrictive group (21.5 vs. 28.8 days, p < 0.001). There was no significant difference in the postoperative medical complications including myocardial infarction and cerebrovascular event. Mortality at postoperative 30, 60, and 90 days was similar between the two groups.
Conclusions
Our blood management protocol involving restrictive strategy combined with preoperative intravenous iron supplementation appears to be effective and safe in HFS of elderly patients.
Go to :

Keywords: Hip fractures, Iron, Allogeneic blood transfusion, Mortality, Complication
The incidence of hip fractures is increasing worldwide along with the increase of osteoporotic elderly population.
12) Most hip fractures require surgery and became a serious socioeconomic burden, because these fractures are associated with high rates of perioperative morbidity and mortality in elderly patients.
3) Although contemporary surgical techniques have been improved, hip fracture surgery (HFS) is associated with substantial perioperative blood loss, which necessitates transfusion.
45) Blood transfusion can result in abnormalities of coagulation status and transfusion-related complications are causes of morbidity and death. It also prolongs hospital stay and increases the risk of hematogenic infections.
46) For these reasons, bloodsaving strategies were introduced.
78)
Traditionally, transfusion has been performed to maintain a blood hemoglobin (Hb) concentration above 10 g/dL (100 g/L) and a hematocrit above 30%.
910) This is called the liberal transfusion strategy. Recently, the comprehensive concept of multimodal patient blood management (PBM) has been recommended to reduce transfusion and transfusion-related complications by World Health Organization (WHO).
111213) Based on the PBM concept, the restrictive transfusion strategy was developed. In that strategy, the patient is transfused when the level of Hb is lower than 8 g/dL or anemic symptoms are present.
14151617) Intravenous supplementation of iron has been known to reduce or prevent red blood cell transfusion in patients undergoing joint arthroplasty.
18)
We followed the traditional liberal strategy with the higher transfusion threshold in HFS patients until May 2009. In June 2009, we adopted the PBM of the restrictive strategy combined with intravenous iron supplementation and thereafter, we have used that concept in HFS patients. In this study, we compared the transfusion rate, amounts of transfusion, postoperative Hb level, duration of hospital stay, morbidity, and mortality between the two strategies after HFS.
METHODS
This retrospective study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-1710/427-103), which waived informed consents. We reviewed the medical records of 1,777 patients who underwent surgery due to a hip fracture (femoral neck or intertrochanteric fracture) at one tertiary referral hospital between May 2003 and June 2014 and were aged more than 60 years at the time of surgery. We excluded 13 patients who had pathologic fractures, 48 patients who had revision operations, and 82 patients who were lost to follow-up before 1 year after the operation. This left 1,634 patients, who were subjects of this study.
In all patients the Hb level was measured at admission. Anemia was defined according to the criteria of the WHO
18): a Hb level less than 12 g/dL in women and less than 13 g/dL in men. The Hb level was measured on the first postoperative day as a routine and repeatedly thereafter if considered necessary on clinical grounds.
The liberal transfusion strategy group (liberal group) included 775 patients who underwent HFS between May 2003 and May 2009. In this group, if the Hb level was below 10 g/dL within 3 days after surgery, blood was transfused as much as needed to maintain the Hb level of 10 g/dL or more.
The restrictive transfusion strategy group (restrictive group) included 859 patients who underwent HFS between June 2009 and June 2014. All patients in this group received intravenously 200 mg (maximum daily dose) of iron supplementation (Venoferrum; Vifor, Glattbrugg, Switzerland) before surgery. If the Hb level was below 8 g/dL or symptoms or signs of acute anemia (e.g., dizziness, chest pain, tachycardia, and persistent hypotension) were present, the patient received transfusion in addition to the iron supplementation before the surgery. After the surgery, the presence of anemic symptoms or signs as well as Hb level was monitored. Patients were transfused postoperatively if the anemic symptoms or signs developed or the Hb level fell below 8 g/dL within 3 postoperative days.
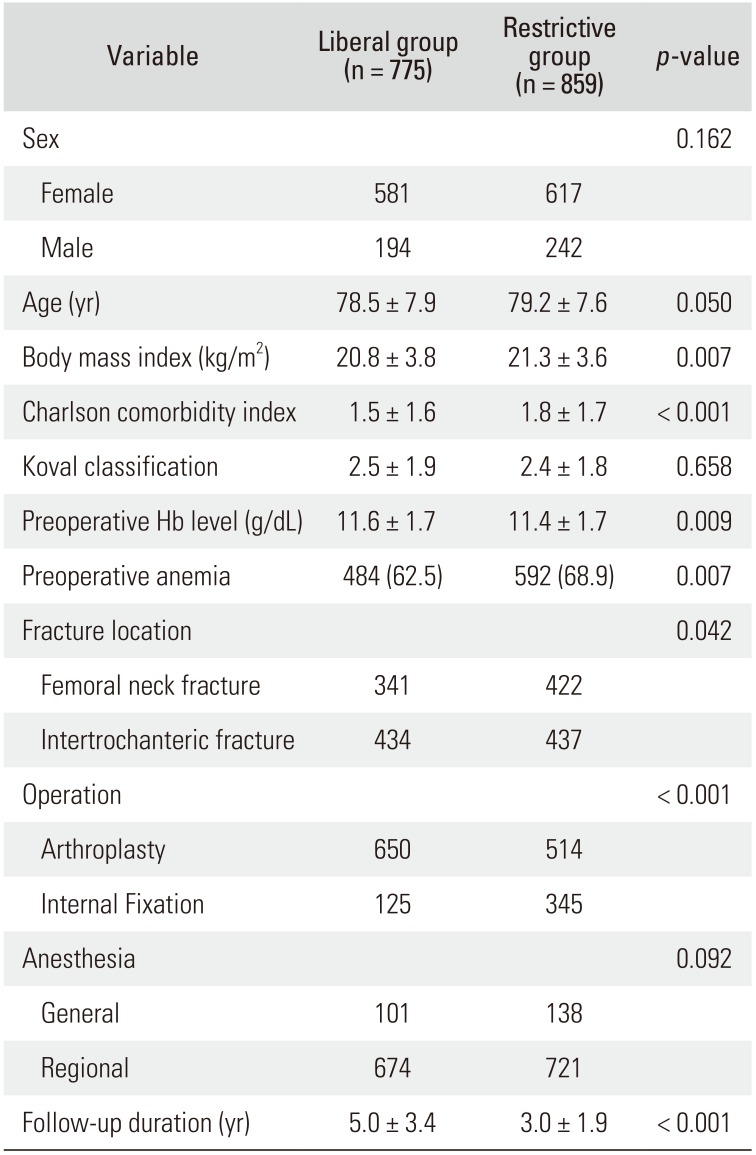
Except for the transfusion management strategy, all surgical procedures and postoperative cares were done in the same manner in the two groups. Basic characteristics of patients of the two groups are presented in
Table 1. Surgeons considered patient's age, presence of osteoporosis, underlying comorbidities, activity level before the injury, and fracture type when they chose the type of operation; internal fixation versus arthroplasty. Because the occurrence of symptomatic venous thromboembolism (VTE) after HFS is rare in East Asian patients even without any thromboprophylaxis,
19) since 2010, all patients have mechanical prophylaxis using intermittent pneumatic compression devices.
20) Only patients who have risk factors for VTE are medicated with thromboprophylaxis agents.
Table 1
Basic Characteristics of Liberal Transfusion Strategy Group (Liberal Group) and Restrictive Transfusion Strategy Group (Restrictive Group)
|
Variable |
Liberal group (n = 775) |
Restrictive group (n = 859) |
p-value |
|
Sex |
|
|
0.162 |
|
Female |
581 |
617 |
|
|
Male |
194 |
242 |
|
|
Age (yr) |
78.5 ± 7.9 |
79.2 ± 7.6 |
0.050 |
|
Body mass index (kg/m2) |
20.8 ± 3.8 |
21.3 ± 3.6 |
0.007 |
|
Charlson comorbidity index |
1.5 ± 1.6 |
1.8 ± 1.7 |
< 0.001 |
|
Koval classification |
2.5 ± 1.9 |
2.4 ± 1.8 |
0.658 |
|
Preoperative Hb level (g/dL) |
11.6 ± 1.7 |
11.4 ± 1.7 |
0.009 |
|
Preoperative anemia |
484 (62.5) |
592 (68.9) |
0.007 |
|
Fracture location |
|
|
0.042 |
|
Femoral neck fracture |
341 |
422 |
|
|
Intertrochanteric fracture |
434 |
437 |
|
|
Operation |
|
|
< 0.001 |
|
Arthroplasty |
650 |
514 |
|
|
Internal Fixation |
125 |
345 |
|
|
Anesthesia |
|
|
0.092 |
|
General |
101 |
138 |
|
|
Regional |
674 |
721 |
|
|
Follow-up duration (yr) |
5.0 ± 3.4 |
3.0 ± 1.9 |
< 0.001 |

After discharge, patients were followed up at 6 weeks, 3 months, 6 months, 9 months, 1 year, and every year thereafter. At postoperative 6 weeks, the level of Hb was measured. We compared the perioperative blood loss (the sum of estimated blood loss during surgery and postoperative drainage), the amount of transfusion, duration of hospital stay, postoperative medical complications (myocardial infarction and cerebrovascular event), and mortality rate between the two groups. The mortality rate was evaluated at postoperative 30, 60, and 90 days. In the restrictive group, we investigated adverse reactions of intravenous iron supplementation, such as hypersensitivity and infusion site reactions.
Statistical Analysis
The Kolmogorov-Smirnov test was first used to estimate the normality, and all continuous variables showed normal distribution. We used chi-square test for categorical data and the Student t-test for continuous data. Statistical analyses were conducted with the SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA). For all other tests, a two-sided p-value < 0.05 was considered significant.
Go to :

RESULTS
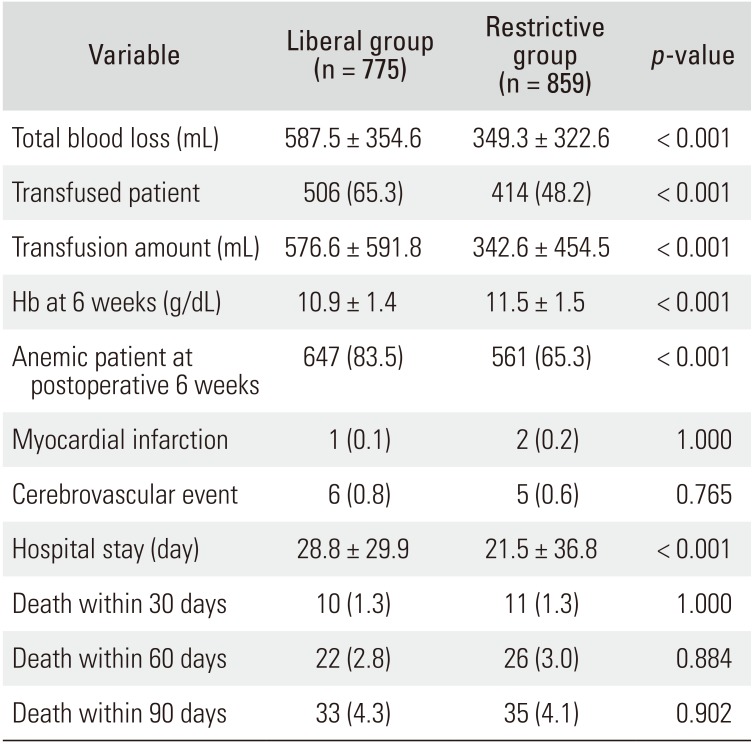
No adverse reaction due to intravenous iron supplementation was identified in the restrictive group. The total blood loss was less in the restrictive group than in the liberal group (349.3 ± 322.6 vs. 587.5 ± 354.6 mL, p < 0.001). Transfusion was performed in 506 patients (65.3%) in the liberal group and in 414 patients (48.2%) in the restrictive group (p < 0.001). The amount of transfused blood was less in the restrictive group than in the liberal group (342.6 ± 454.5 vs. 576.6 ± 591.8 mL, p < 0.001).
At 6 weeks after the surgery, in the restrictive group, the mean Hb was higher (11.5 vs. 10.9 g/dL,
p < 0.001), and the proportion of anemia was lower (65.3% vs. 83.5%). The mean hospital stay was shorter in the restrictive group (21.5 vs. 28.8 days,
p < 0.001). There was no significant difference in medical complications. The mortality at 30, 60, and 90 days were similar between the two groups (
Table 2).
Table 2
Comparison between Liberal Transfusion Strategy Group (Liberal Group) and Restrictive Transfusion Strategy Group (Restrictive Group)
|
Variable |
Liberal group (n = 775) |
Restrictive group (n = 859) |
p-value |
|
Total blood loss (mL) |
587.5 ± 354.6 |
349.3 ± 322.6 |
< 0.001 |
|
Transfused patient |
506 (65.3) |
414 (48.2) |
< 0.001 |
|
Transfusion amount (mL) |
576.6 ± 591.8 |
342.6 ± 454.5 |
< 0.001 |
|
Hb at 6 weeks (g/dL) |
10.9 ± 1.4 |
11.5 ± 1.5 |
< 0.001 |
|
Anemic patient at postoperative 6 weeks |
647 (83.5) |
561 (65.3) |
< 0.001 |
|
Myocardial infarction |
1 (0.1) |
2 (0.2) |
1.000 |
|
Cerebrovascular event |
6 (0.8) |
5 (0.6) |
0.765 |
|
Hospital stay (day) |
28.8 ± 29.9 |
21.5 ± 36.8 |
< 0.001 |
|
Death within |
30 days 10 (1.3) |
11 (1.3) |
1.000 |
|
Death within |
60 days 22 (2.8) |
26 (3.0) |
0.884 |
|
Death within |
90 days 33 (4.3) |
35 (4.1) |
0.902 |

Go to :

DISCUSSION
Our study showed that the restrictive transfusion strategy (PBM) combined with preoperative intravenous iron supplementation could reduce the rate and amount of transfusion after HFS without increasing perioperative morbidity and mortality. Furthermore, postoperative Hb level at 6 weeks was higher in the restrictive group. Blood transfusions are frequently required in HFS of elderly patients. Yet, the Hb threshold at which blood transfusion is necessary remains controversial and only limited number of studies on this issue are available.
172122) The reduced transfusion rate could be derived from the change of transfusion criteria (Hb level, from 10 g/dL to 8 g/dL). But, the perioperative morbidity and mortality did not increase in the restrictive group.
Some studies have demonstrated the effects of postoperative intravenous iron supplementation on reduced requirement of blood transfusion or on the recovery of Hb.
23242526) To our understanding, the current study is the first one that evaluated the effects of restrictive transfusion strategy combined with preoperative iron supplementation. Considering the time required for erythropoiesis after iron supplementation, our results suggest that our protocol would be more useful in HFS than the restrictive strategy alone. Interestingly, the Hb level was higher at 6 weeks in patients who were given intravenous iron injection preoperatively.
The use of intravenous iron supplementation has several concerns including hypersensitivity, cardiovascular events, and infusion site reaction.
27) However, a systematic review concluded that intravenous iron supplementation is not associated with increased risk of drug reactions or infections.
28) In our patients, we did not encounter any ironrelated adverse reactions.
Our study is not a randomized clinical study, but it is a retrospective study comparing with a historical control group. In addition, the efficacy of perioperative iron supplementation and restrictive strategy were already reported;
232425262930) thus, a randomized clinical trial would not be ethically justified. Although age, sex, and body mass index were similar between both groups, other factors including type of surgery such as arthroplasty might have affected the outcomes. Our blood management protocol consisting of the restrictive transfusion strategy combined with preoperative intravenous iron supplementation was effective and safe in HFS of elderly patients.
Go to :

ACKNOWLEDGEMENTS
We appreciate research grant from JW pharmaceutical.
Go to :

Notes
Go to :

References
1. Ha YC, Kim TY, Lee A, et al. Current trends and future projections of hip fracture in South Korea using nationwide claims data. Osteoporos Int. 2016; 27(8):2603–2609. PMID:
27112763.

2. Lee YK, Kim JW, Lee MH, Moon KH, Koo KH. Trend in the age-adjusted incidence of hip fractures in South Korea: systematic review. Clin Orthop Surg. 2017; 9(4):420–423. PMID:
29201294.

3. Gronskag AB, Romundstad P, Forsmo S, Langhammer A, Schei B. Excess mortality after hip fracture among elderly women in Norway: the HUNT study. Osteoporos Int. 2012; 23(6):1807–1811. PMID:
22068386.
4. Foss NB, Kehlet H. Hidden blood loss after surgery for hip fracture. J Bone Joint Surg Br. 2006; 88(8):1053–1059. PMID:
16877605.

5. Swain DG, Nightingale PG, Patel JV. Blood transfusion requirements in femoral neck fracture. Injury. 2000; 31(1):7–10.

6. Foss NB, Kristensen MT, Kehlet H. Anaemia impedes functional mobility after hip fracture surgery. Age Ageing. 2008; 37(2):173–178. PMID:
18349013.

7. Carson JL, Noveck H, Berlin JA, Gould SA. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002; 42(7):812–818. PMID:
12375651.
8. Sheikh HQ, Hossain FS, Aqil A, Akinbamijo B, Mushtaq V, Kapoor H. A comprehensive analysis of the causes and predictors of 30-day mortality following hip fracture surgery. Clin Orthop Surg. 2017; 9(1):10–18. PMID:
28261422.

9. Wang JK, Klein HG. Red blood cell transfusion in the treatment and management of anaemia: the search for the elusive transfusion trigger. Vox Sang. 2010; 98(1):2–11. PMID:
19682346.

10. Adams RC, Lundy JS. Anesthesia in cases of poor surgical risk: some suggestions for decreasing the risk. Anesthesiology. 1942; 3(5):603–607.
11. Jassil FC, Carnemolla A, Kingett H, et al. Protocol for a 1-year prospective, longitudinal cohort study of patients undergoing Roux-en-Y gastric bypass and sleeve gastrectomy: the BARI-LIFESTYLE observational study. BMJ Open. 2018; 8(3):e020659.

12. Shander A, Van Aken H, Colomina MJ, et al. Patient blood management in Europe. Br J Anaesth. 2012; 109(1):55–68. PMID:
22628393.

13. Althoff FC, Neb H, Herrmann E, et al. Multimodal patient blood management program based on a three-pillar strategy: a systematic review and meta-analysis. Ann Surg. 2019; 269(5):794–804. PMID:
30418206.
14. Bisbe E, Molto L, Arroyo R, Muniesa JM, Tejero M. Randomized trial comparing ferric carboxymaltose vs oral ferrous glycine sulphate for postoperative anaemia after total knee arthroplasty. Br J Anaesth. 2014; 113(3):402–409. PMID:
24780615.

15. Suh YS, Nho JH, Choi HS, Ha YC, Park JS, Koo KH. A protocol avoiding allogeneic transfusion in joint arthroplasties. Arch Orthop Trauma Surg. 2016; 136(9):1213–1226. PMID:
27450193.

16. Carson JL, Sieber F, Cook DR, et al. Liberal versus restrictive blood transfusion strategy: 3-year survival and cause of death results from the FOCUS randomised controlled trial. Lancet. 2015; 385(9974):1183–1189. PMID:
25499165.

17. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011; 365(26):2453–2462. PMID:
22168590.

18. Persson P, Gagnemo-Persson R, Chen D, et al. Gastrectomy causes bone loss in the rat: is lack of gastric acid responsible? Scand J Gastroenterol. 1993; 28(4):301–306. PMID:
8488363.

19. Kang BJ, Lee YK, Kim HJ, Ha YC, Koo KH. Deep venous thrombosis and pulmonary embolism are uncommon in East Asian patients after total hip arthroplasty. Clin Orthop Relat Res. 2011; 469(12):3423–3428. PMID:
21748508.

20. Jo WL, Lee YK, Ha YC, Lee KM, Kang BJ, Koo KH. Preventing venous thromboembolism with use of intermittent pneumatic compression after total hip arthroplasty in Korean patients. J Korean Med Sci. 2016; 31(8):1319–1323. PMID:
27478345.

21. Lombardi G, Rizzi E, Zocca N, Inzoli MR. Epidemiology of anemia in older patients with hip fracture. J Am Geriatr Soc. 1996; 44(6):740–741. PMID:
8642178.

22. Yoon BH, Ko YS, Jang SH, Ha JK. Feasibility of hip fracture surgery using a no transfusion protocol in elderly patients: a propensity score-matched cohort study. J Orthop Trauma. 2017; 31(8):414–419. PMID:
28459771.

23. Khalafallah AA, Yan C, Al-Badri R, et al. Intravenous ferric carboxymaltose versus standard care in the management of postoperative anaemia: a prospective, open-label, randomised controlled trial. Lancet Haematol. 2016; 3(9):e415–e425. PMID:
27570088.

24. Munoz M, Garcia-Erce JA, Cuenca J, Bisbe E, Naveira E. AWGE (Spanish Anaemia Working Group). On the role of iron therapy for reducing allogeneic blood transfusion in orthopaedic surgery. Blood Transfus. 2012; 10(1):8–22. PMID:
22153694.
25. Kim SK, Seo WY, Kim HJ, Yoo JJ. Postoperative intravenous ferric carboxymaltose reduces transfusion amounts after orthopedic hip surgery. Clin Orthop Surg. 2018; 10(1):20–25. PMID:
29564043.

26. Kotze A, Carter LA, Scally AJ. Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012; 108(6):943–952. PMID:
22593128.
27. Walters BA, Van Wyck DB. Benchmarking iron dextran sensitivity: reactions requiring resuscitative medication in incident and prevalent patients. Nephrol Dial Transplant. 2005; 20(7):1438–1442. PMID:
15840683.

28. Avni T, Bieber A, Grossman A, Green H, Leibovici L, Gafter-Gvili A. The safety of intravenous iron preparations: systematic review and meta-analysis. Mayo Clin Proc. 2015; 90(1):12–23. PMID:
25572192.
29. Munoz M, Naveira E, Seara J, Cordero J. Effects of postoperative intravenous iron on transfusion requirements after lower limb arthroplasty. Br J Anaesth. 2012; 108(3):532–534. PMID:
22337966.
30. Munoz M, Gomez-Ramirez S, Cuenca J, et al. Very-shortterm perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion. 2014; 54(2):289–299. PMID:
23581484.

Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download