초록
Background
Magnetic resonance (MR) images are useful for diagnosing myopathy. The purpose of this study was to determine the usefulness of lower-limb MR images in Korean patients with distal myopathy.
Methods
We reviewed medical records in the myopathy database from January 2002 to Oc-tober 2016. We selected 21 patients from 91 unrelated families with distal myopathy: four with GNE myopathy, 11 with dysferlinopathy, and six with ADSSL1 myopathy.
Results
Ten (48%) of the 21 patients were men. The ages of the participants at symptom on-set and imaging were 19.2 ± 9.5 and 30.4 ± 9.0 years (mean ± standard deviation), respectively. Their grade on the modified Gardner-Medwin and Walton grade was 3.3 ± 1.7. The strength grade of the knee extensors was not correlated with the Mercuri scale for the quadriceps (r = –0.247, p = 0.115). However, the Medical Research Council grades of the knee flexors, ankle dorsiflexors, and ankle plantar flexors were significantly correlated with the Mercuri scale ratings of the knee flexors (r = –0.497, p = 0.001), tibialis anterior (r = –0.727, p < 0.001), and ankle plantar flexors (r = –0.620, p < 0.001), respectively. T1-weighted MR images showed character-istic fatty replacement patterns that were consistent with the causative genes. Unsupervised hierarchical clustering of the Mercuri scale showed that the main factors contributing to the dichotomy were the causative gene and the clinical severity.
Go to : 
REFERENCES
1.Bonne G., Rivier F., Hamroun D. The 2018 version of the gene ta-ble of monogenic neuromuscular disorders (nuclear genome). Neuromuscul Disord. 2017. 27:1152–1183.

2.Park HJ., Shin HY., Kim S., Kim SH., Lee Y., Lee JH, et al. Distal myopathy with ADSSL1 mutations in Korean patients. Neuromuscul Disord. 2017. 27:465–472.

3.Straub V., Carlier PG., Mercuri E. TREAT-NMD workshop: pattern recognition in genetic muscle diseases using muscle MRI: 25–26 February 2011, Rome, Italy. Neuromuscul Disord. 2012. 22(Suppl 2):S42–S53.
4.Díaz-Manera J., Llauger J., Gallardo E., Illa I. Muscle MRI in muscular dystrophies. Acta Myol. 2015. 34:95–108.
5.Kinali M., Arechavala-Gomeza V., Cirak S., Glover A., Guglieri M., Feng L, et al. Muscle histology vs MRI in Duchenne muscular dystrophy. Neurology. 2011. 76:346–353.

6.Fanin M., Angelini C. Muscle pathology in dysferlin deficiency. Neuropathol Appl Neurobiol. 2002. 28:461–470.

7.Fischer D., Clemen CS., Olivé M., Ferrer I., Goudeau B., Roth U, et al. Different early pathogenesis in myotilinopathy compared to primary desminopathy. Neuromuscul Disord. 2006. 16:361–367.

8.Jungbluth H., Davis MR., Müller C., Counsell S., Allsop J., Chattopad-hyay A, et al. Magnetic resonance imaging of muscle in congen-ital myopathies associated with RYR1 mutations. Neuromuscul Disord. 2004. 14:785–790.

9.Eisenberg I., Avidan N., Potikha T., Hochner H., Chen M., Olender T, et al. The UDP-N-acetylglucosamine 2-epimerase/N-acetylman-nosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet. 2001. 29:83–87.

10.Sim JE., Park HJ., Shin HY., Nam TS., Kim SM., Choi YC. Clinical characteristics and molecular genetic analysis of Korean patients with GNE myopathy. Yonsei Med J. 2013. 54:578–582.

11.Liu J., Aoki M., Illa I., Wu C., Fardeau M., Angelini C, et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet. 1998. 20:31–36.

12.Park HJ., Hong JM., Suh GI., Shin HY., Kim SM., Sunwoo IN, et al. Het-erogeneous characteristics of Korean patients with dysferlinopathy. J Korean Med Sci. 2012. 27:423–429.

Go to : 
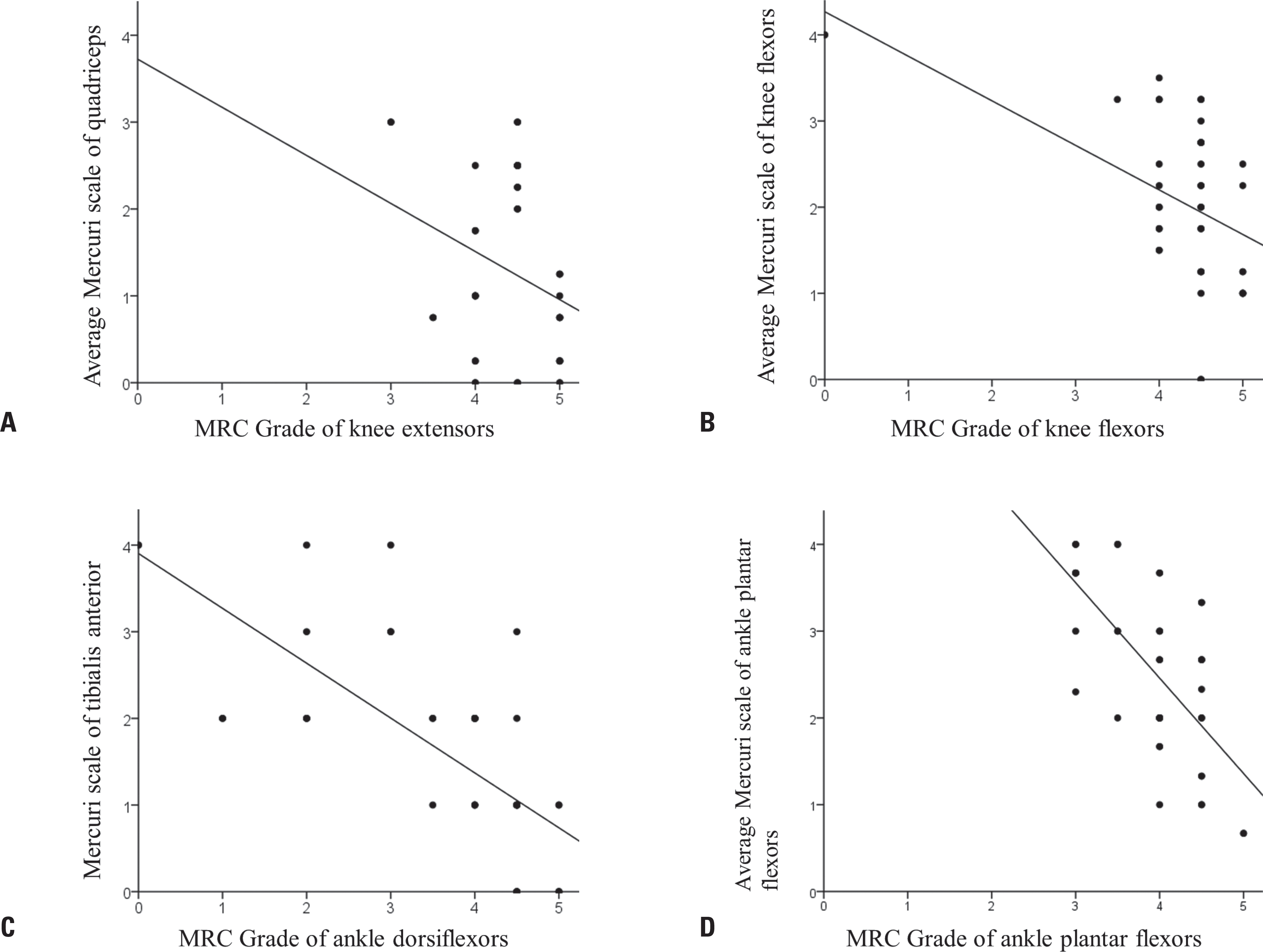 | Fig. 1.Relationship between muscle strength and the degree of fatty replacement observed in lower-limb magnetic resonance (MR) images. (A) The Mercuri scale rating of the quadriceps (rectus femoris, vastus lateralis, vastus medialis, and vastus intermedius) was not significantly correlated with the Medical Research Council (MRC) grade of the knee extensors (r = –0.247, p = 0.115). (B) The Mercuri scale rating of the knee flexors (semitendinosus, semimembranosus, and the short and long heads of biceps femoris) was significantly correlated with the MRC grade of the knee flexors (r = –0.497, p = 0.001). (C) The Mercuri scale rating of the tibialis anterior was significantly correlated with the MRC grade of the ankle dorsiflexors (r = –0.727, p < 0.001). (D) The Mercuri scale rating of the ankle plantar flexors (soleus and medial/lateral gastrocnemius) was significantly correlated with the MRC grade of the ankle plantar flexors (r = –0.620, p < 0.001). |
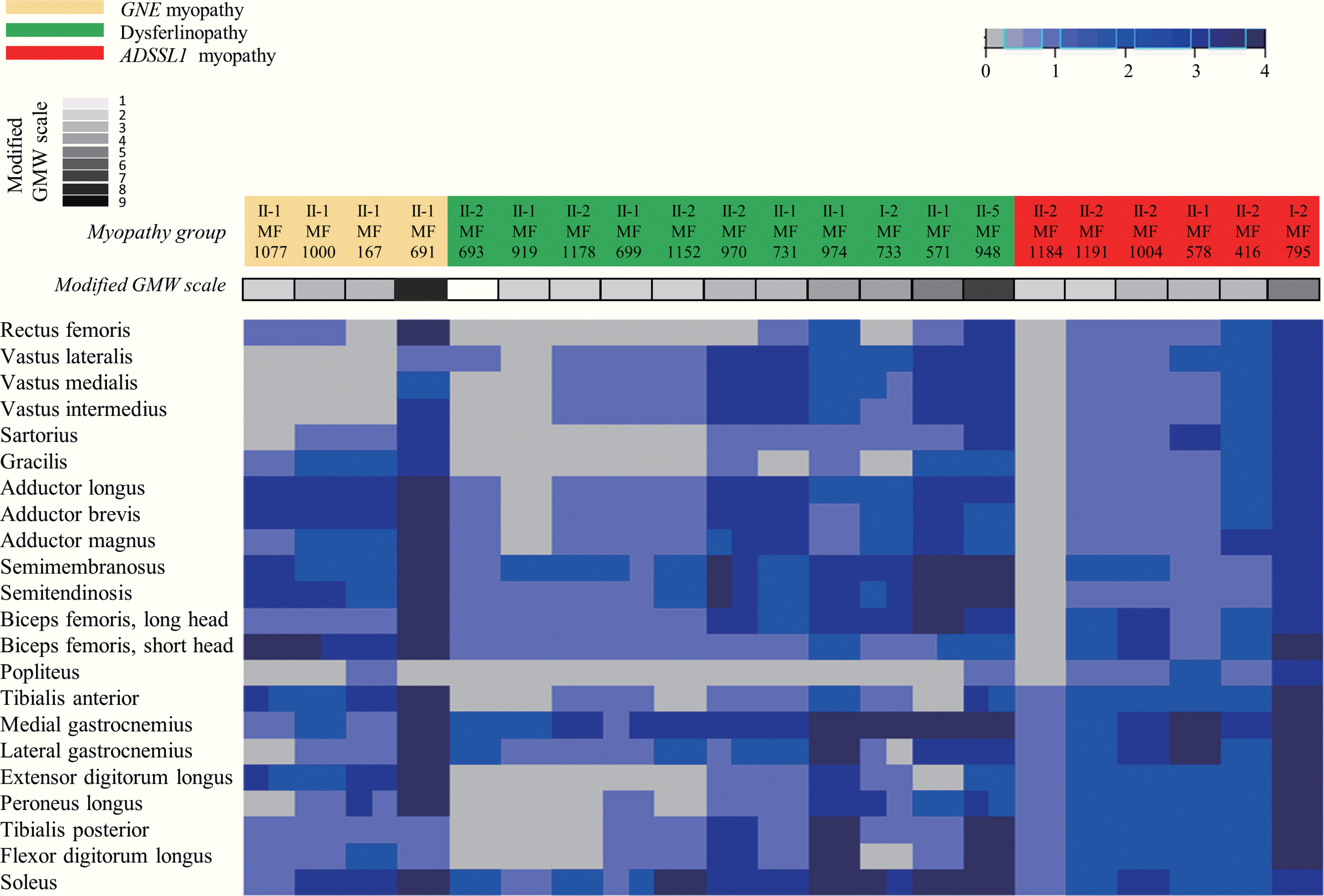 | Fig. 2.Heat map representing muscle degeneration. GNE myopathy was characterized by the early involvement of the tibialis anterior and the short head of the biceps femoris, with relative sparing of the vastus lateralis. Dysferlinopathy showed the early involvement of the posterior compartment of the calf and thigh muscles, with relative sparing of the sartorius, gracilis, and rectus femoris. ADSSL1 myopathy showed fatty replacements of the gastrocnemius that began at the early stages. Each column in the heat map corresponds to one patient. Each row corresponds to one muscle, in de-scending order from cranial to caudal. A gray–blue–midnight-blue gradient in the heat map indicates increasing fatty substitution (legend at the top). The two rows at the left of the heat map denote individual clinical features: the myopathy group and grade on the modified Gardner-Medwin and Walton (GMW) grade, color coded as indicated in the legend on the left. |
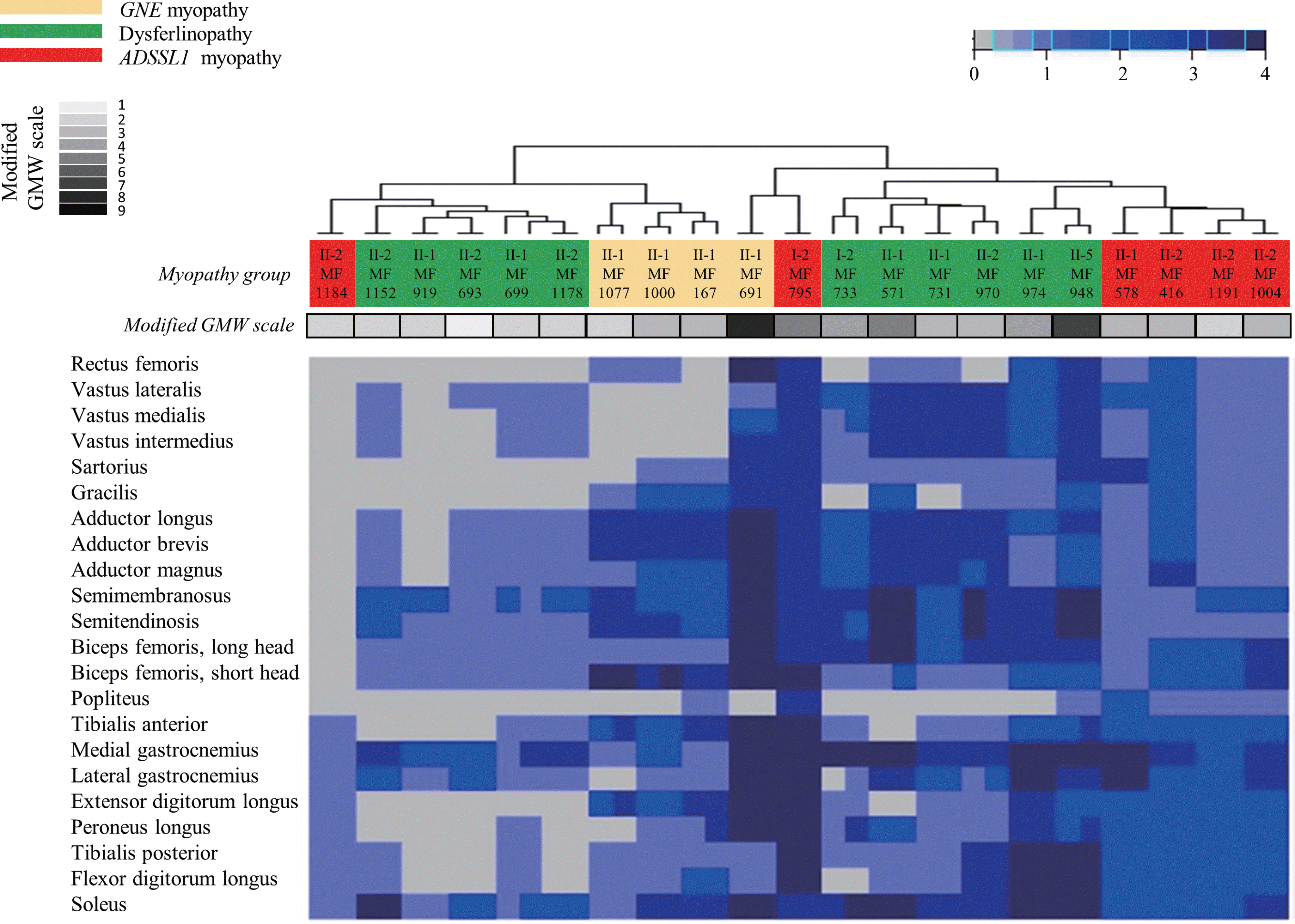 | Fig. 3.Hierarchically clustered heat map of Mercuri scale ratings from magnetic resonance (MR) images from 21 patients with distal myopathy. Hierar-chical clustering of the Mercuri scale ratings showed that the included patients could be divided into two distinct subpopulations: one with mild fatty replacement and the other with moderate/severe fatty replacement. The main factors contributing to this dichotomy were the causative gene and the clinical severity (as assessed using the modified GMW grade). Each column corresponds to one patient, and they are hierarchically clustered (dendrogram at the top) based solely on MR imaging data (using the Mercuri scale). Each row in the heat map corresponds to one muscle, in de-scending order from cranial to caudal. A gray-blue-midnight-blue gradient in the heat map indicates increasing fatty substitution (legend at the top). The two rows at the left of the heat map denote individual clinical features (not used in the hierarchical clustering algorithm): the myopathy group and grade on the modified GMW grade, color coded as indicated in the legend on the left. GMW, Gardner-Medwin and Walton. |
Table 1.
Clinical and genetic data of the patients with distal myopathy




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download