Abstract
Purpose
To investigate the location of retinal nerve fiber layer defects (RNFLDs) in open-angle glaucoma and the differences in systemic and ocular factors between superotemporal and inferotemporal RNFLDs.
Methods
We performed a retrospective review of the 2008 to 2012 data from the Korea National Health and Nutrition Examination Survey. Subjects aged ≥19 years with an evaluable fundus photograph of at least one eye were enrolled, and open-angle glaucoma was diagnosed according to modified International Society of Geographical and Epidemiological Ophthalmology criteria. In subjects with open-angle glaucoma, locations of RNFLDs were evaluated, and systemic and ocular factors were compared between the bilateral superotemporal RNFLD group and bilateral inferotemporal RNFLD group.
Results
A total of 534 subjects had open-angle glaucoma with RNFLDs. The unilateral inferotemporal region (25.8%) was the most common location for RNFLDs, followed by the unilateral superotemporal region (24.4%). Multivariate analysis revealed that hypertension was more significantly associated (p = 0.048) with the bilateral superotemporal RNFLD group than with the bilateral inferotemporal RNFLD group.
Glaucoma is a progressive disease characterized by optic neuropathy with retinal nerve fiber layer defects (RNFLDs). Several epidemiological studies have revealed that hypertension, diabetes, history of thyroid disease, high intraocular pressure (IOP), myopia, and long axial length are systemic and ocular risk factors for open-angle glaucoma [123456]. Among Koreans, older age, male sex, high IOP, myopia, hypertension, and non-overweight status showed positive associations with primary open-angle glaucoma [7].
The retinal nerve fiber is a bundle of axons extending from the retinal ganglion cell to the optic disc. The RNFL located in the temporal area of the optic disc is divided into the superior and inferior RNFLs by the horizontal raphe [8].
Several studies have reported differences in characteristics of glaucomatous damage according to the area. The superior visual field (VF) is more frequently involved than is the inferior VF [910], and the progression rate is faster in the superior VF than in the inferior VF [1112]. In optical coherence tomography-based retinal nerve fiber layer analysis, progression is most frequently observed in the inferotemporal area in glaucoma [12]. Hood et al. [13] reported that the inferior region of the macula is more susceptible to glaucomatous damage than is the superior region because of structural differences.
In the present study, we evaluated the locations of RNFLDs and assessed the differences in systemic and ocular factors between superior and inferior RNFLDs in open-angle glaucoma on the basis of data derived from the Korea National Health and Nutrition Examination Survey (KNHANES).
The institutional review board of Kangdong Sacred Heart Hospital approved this study (2017-11-005), which adhered to the tenets of the Declaration of Helsinki. Written informed consent was waived due to the retrospective nature of the study.
The KNHANES is a nationwide population-based cross-sectional study conducted by the Ministry of Health and Welfare of the Republic of Korea. It uses a complex, stratified, multistage, clustered-probability survey based on National Census Data and involves representative, civilian, noninstitutionalized Korean citizens residing in Korea. The data from the KNHANES can be considered as representing the entire Korean population.
The Korean Ophthalmological Society has participated in the survey since the latter half of 2008. The present study was based on the data collected from 2008 to 2012 (KNHANES IV–V). The target populations were 12,528, 12,722, 10,938, 10,589, and 10,069 in 2008, 2009, 2010, 2011, and 2012, respectively. In the present study, subjects aged ≥19 years with an evaluable fundus photograph of at least one eye were enrolled.
A detailed explanation of the KNHANES has been provided previously [14]. In brief, the KNHANES consists of three contents: health interview, health examination, and nutritional survey. The health interview and health examination are performed by interviewers and trained medical staff at mobile examination centers. The health interview questionnaire consists of questions regarding demographic variables, as well as current and past medical conditions. The health examination consists of measurements of height, weight, waist circumference, and systolic and diastolic blood pressure; blood tests (fasting glucose, glycosylated hemoglobin, total cholesterol, high-density lipoprotein cholesterol, triglyceride, hemoglobin, and hematocrit); and ophthalmic examinations.
The ophthalmic examinations were performed by an ophthalmologist trained by the Korean Ophthalmological Society National Epidemiological Survey Committee for quality control. Visual acuity on the logMAR scale (Jin's Vision chart; Seoul, Korea) and refractive error determined by automatic refractometry (KR8800; Topcon, Tokyo, Japan) were measured. Slit-lamp examination was performed for anterior segment evaluation, including assessment of peripheral anterior chamber depth using the van Herick methods. IOP was measured using Goldmann applanation tonometry. Fundus photographs were acquired using a nonmydriatic fundus camera (TRC-NW6S, Topcon) in participants aged 19 years and older. VF tests using frequency-doubling technology (FDT) perimetry were also performed using the screening program N30-1 (Humphrey Matrix FDT perimetry; Carl Zeiss Meditec, Dublin, CA, USA) in participants with elevated IOP (≥22 mmHg) or glaucomatous optic disc appearance. Glaucomatous optic disc was determined on the basis of the following criteria: (1) horizontal or vertical cup-to-disc ratio (C/D ratio) ≥0.7, (2) violation of the ISNT rule (neuroretinal rim thickness order: inferior > superior > nasal > temporal), (3) presence of optic disc hemorrhage, and (4) presence of a RNFLD.
The detailed method of fundus photography evaluation has been described previously [14]. In brief, two blinded ophthalmologists from different institutes evaluated the fundus photographs. Any discrepancy was adjudicated by a third group of glaucoma specialists. The C/D ratio, ISNT rule, disc hemorrhage, and RNFLDs were recorded.
Open-angle glaucoma was defined as presence of an open angle determined by the van Herick method with the following criteria of the modified International Society of Geographical and Epidemiological Ophthalmology. Category 1 criteria applied to subjects with reliable FDT perimetry (fixation error and false-positive error ≤1). Subjects had to show abnormal FDT perimetry compatible with optic disc appearance or an RNFLD and satisfy one of the following criteria: (1) vertical or horizontal C/D ratio ≥0.7 or asymmetry of C/D ratio ≥0.2, (2) presence of disc hemorrhage, or (3) presence of an RNFLD. Category 2 criteria were applied to subjects with unreliable FDT perimetry (fixation error or false-positive error ≥1) or absence of FDT perimetry results. Subjects had to satisfy one of the following criteria: (1) vertical or horizontal C/D ratio ≥0.9 or asymmetry of C/D ratio ≥0.3 or (2) presence of an RNFLD compatible with optic disc appearance.
The KNHANES has a stratified, multistage, clustered-probability design. The complex sample analysis was performed in accordance with the statistical guidelines of the Korea Centers for Disease Control and Prevention.
The prevalence of open-angle glaucoma was expressed as a weight-adjusted estimation of percentages with a 95% confidence interval (CI). The RNFLD location in subjects with open-angle glaucoma who had an RNFLD compatible with FDT perimetry (category 1 criteria) or optic disc appearance (category 2 criteria) was expressed as a weight-adjusted estimation of percentages with a 95% CI. Differences between bilateral superotemporal and bilateral inferotemporal RNFLD groups were evaluated using a generalized linear model for complex samples and the chi-square test for complex samples. Univariate and multivariate logistic regression analyses were performed to determine systemic and ocular factors affecting the location of glaucomatous damage. Ocular parameters of the right eye were chosen in the statistical analysis. Statistical significance was defined as a p < 0.05.
In total, 56,846 subjects were selected for the KNHANES IV–V (2008 to 2012), and 45,811 (80.6%) subjects attended the health interviews and health examinations. Among them, 28,687 subjects aged ≥19 years with an evaluable fundus photograph of at least one eye were enrolled in this study.
The number of subjects with open-angle glaucoma was 813, and the estimated prevalence in the Korean population aged ≥19 years was 2.5% (95% CI, 2.3 to 2.7). Among these 813 subjects, 534 had at least one RNFLD compatible with FDT perimetry or optic disc appearance.
Table 1 shows RNFLD location in the 534 subjects with open-angle glaucoma. The unilateral inferotemporal region was the most common location of defects, followed by the unilateral superotemporal region (Fig. 1).
Among a total of 997 RNFLDs, 503 (50.5%) were located in the superotemporal area, and 494 (49.5%) were located in the inferotemporal area.
Table 2 shows the systemic and ocular parameters of the bilateral superotemporal RNFLD group (42 subjects) and bilateral inferotemporal RNFLD group (36 subjects). Systolic blood pressure and diastolic blood pressure were significantly higher in the bilateral superotemporal RNFLD group than in the bilateral inferotemporal RNFLD group. Hypertension was also more prevalent in the bilateral superotemporal RNFLD group, with borderline significance (p = 0.050).
Univariate analysis revealed that the bilateral superotemporal RNFLD group had higher systolic blood pressure (p = 0.027) and higher diastolic blood pressure (p = 0.029) (Table 3). Variables with a p-value less than 0.10 after the univariate analysis were subsequently included in the multivariate analysis. Some of the factors were excluded considering the multicollinearity and clinical significance of the factors; hypertension and vertical C/D ratio were included. The multivariate analysis indicated that bilateral superotemporal RNFLDs were associated with hypertension (p = 0.048) (Table 4).
In this study, the estimated prevalence of open-angle glaucoma was 2.5% (95% CI, 2.3 to 2.7) in a Korean population aged ≥19 years and 3.3% (95% CI, 3.0 to 3.7) in a Korean population aged ≥40 years. These prevalence values were lower than those reported in a previous study using identical KNHANES data (2008 to 2012) (2.6%, 3.4%), because open-angle glaucoma alone was analyzed in this study, whereas both angle-closure glaucoma and open-angle glaucoma were analyzed without distinction in the previous study [14].
In the present study, the most common location of RNFLDs was the unilateral inferotemporal region (26.8%), and the prevalence was similar to that in the second most common location, the unilateral superotemporal region (24.4%). Among a total of 997 RNFLDs, 503 (50.5%) were superotemporal RNFLDs, and 494 (49.5%) were inferotemporal RNFLDs. In previous studies, the superior VF was more frequently affected than the inferior VF [9101213]. When considering the spatial relationship between RNFLDs and the VF, we assumed that inferotemporal RNFLDs were more prevalent than superotemporal RNFLDs. However, in this study, no significant difference was observed between the prevalence of superotemporal and inferotemporal RNFLDs. The discrepancies among these findings may reflect the differences in study population characteristics and in the determination of the location of glaucomatous damage.
To assess the effects of systemic and ocular factors on superotemporal or inferotemporal glaucomatous damage in open-angle glaucoma, we compared the bilateral superotemporal RNFLD group and the bilateral inferotemporal RNFLD group. We found that hypertension was more common in the bilateral superotemporal RNFLD group than in the bilateral inferotemporal RNFLD group. It is possible that the systemic factors affecting both eyes show more significant differences between superior and inferior RNFLDs than do ocular factors that have local effects.
In several epidemiological studies, hypertension has been revealed as a risk factor for open-angle glaucoma. In the Blue Mountains Eye Study, the prevalence of systemic hypertension was higher in participants with glaucoma (65.7%) than in those without glaucoma (45.4%) [15]. In a Korean population-based study using the KNHANES data (2008 to 2011), open-angle glaucoma was significantly associated with hypertension, dyslipidemia, cerebral stroke, and diabetes mellitus on univariate analysis, but after multivariate analysis, only hypertension remained significant [7]. Several hypotheses have been suggested on the effect of systemic hypertension on glaucoma development. One hypothesis is that systemic hypertension increases IOP. This positive association between increased IOP and elevated blood pressure has been confirmed in previous studies [16171819202122] and may be due to enhancement of aqueous production [23] or elevation of episcleral venous pressure [24]. Another hypothesis is that systemic hypertension reduces ocular perfusion to the optic nerve head, possibly due to direct microvascular damage resulting from systemic hypertension, such as retinal vascular narrowing [2526] or impairment of posterior ciliary circulation autoregulation [2527].
We assume a higher risk of lower perfusion pressure in the superior optic nerve head than in the inferior optic nerve head. In a similar example, inferior VF defects are most common in nonarteritic ischemic optic neuropathy caused by vascular insufficiency and leading to optic nerve head ischemia [28]. In a recent study on patients with type 2 diabetes, superior RNFLDs were shown to be more prevalent than inferior RNFLDs. Diabetes is a well-known cause of vascular endothelial dysfunction. That study suggested that this vascular insufficiency causes RNFLDs, and because of the influence of gravity, RNFLDs were more prevalent in the superior area [29]. Further studies are needed to understand the effect of hypertension on the development of glaucoma and why the prevalence of hypertension is different in patients with superior and inferior RNFLDs.
Our study was limited by its cross-sectional design. All parameters were measured and recorded only on the examination date. Therefore, our findings may not reflect potential fluctuations in blood pressure, blood test parameters, or IOP. However, this and other limitations notwithstanding, the strength of this study is that the KNHANES is a nationally representative survey based on a large sample size.
In conclusion, to our knowledge, this is the first study on systemic factors associated with the location of RNFLDs. Hypertension was more significantly associated with superior RNFLDs than with inferior RNFLDs. The results of this study increase understanding of the pathophysiology of glaucoma.
Figures and Tables
 | Fig. 1Participation flow chart from the Korea National Health and Nutrition Examination Survey (KNHANES) 2008 to 2012. ISGEO = International Society of Geographical and Epidemiological Ophthalmology; FDT = frequency-doubling technology. |
Table 2
Baseline patient characteristics
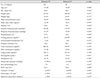
ST = superotemporal retinal nerve fiber layer defect; IT = inferotemporal retinal nerve fiber layer defect; HDL = high-density lipoprotein; ISNT rule = the thickness of disc rims beginning with the inferior rim followed by the superior rim, the nasal rim, and the temporal rim; C/D ratio = cup-to-disc ratio.
*Weight-adjusted estimations of numerical values are shown as mean; †Generalized linear model for complex samples; ‡Weight-adjusted estimations of percentage in each group; §Chi-square test for complex samples.
References
1. Lee AJ, Rochtchina E, Wang JJ, et al. Open-angle glaucoma and systemic thyroid disease in an older population: The Blue Mountains Eye Study. Eye (Lond). 2004; 18:600–608.

2. Mitchell P, Lee AJ, Rochtchina E, Wang JJ. Open-angle glaucoma and systemic hypertension: the blue mountains eye study. J Glaucoma. 2004; 13:319–326.
3. Mitchell P, Smith W, Chey T, Healey PR. Open-angle glaucoma and diabetes: the Blue Mountains eye study, Australia. Ophthalmology. 1997; 104:712–718.
4. Zhou M, Wang W, Huang W, Zhang X. Diabetes mellitus as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. PLoS One. 2014; 9:e102972.

5. Suzuki Y, Iwase A, Araie M, et al. Risk factors for open-angle glaucoma in a Japanese population: the Tajimi Study. Ophthalmology. 2006; 113:1613–1617.
6. Vijaya L, Rashima A, Panday M, et al. Predictors for incidence of primary open-angle glaucoma in a South Indian population: the Chennai eye disease incidence study. Ophthalmology. 2014; 121:1370–1376.
7. Kim KE, Kim MJ, Park KH, et al. Prevalence, awareness, and risk factors of primary open-angle glaucoma: Korea National Health and Nutrition Examination Survey 2008–2011. Ophthalmology. 2016; 123:532–541.
9. Heijl A, Lundqvist L. The frequency distribution of earliest glaucomatous visual field defects documented by automatic perimetry. Acta Ophthalmol (Copenh). 1984; 62:658–664.

10. Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981; 99:635–649.
11. Cho HK, Kee C. Comparison of the progression rates of the superior, inferior, and both hemifield defects in normal-tension glaucoma patients. Am J Ophthalmol. 2012; 154:958–968.

12. Pereira ML, Kim CS, Zimmerman MB, et al. Rate and pattern of visual field decline in primary open-angle glaucoma. Ophthalmology. 2002; 109:2232–2240.

13. Hood DC, Raza AS, de Moraes CG, et al. Glaucomatous damage of the macula. Prog Retin Eye Res. 2013; 32:1–21.

14. Na KI, Jeoung JW, Lee WJ, et al. Prevalence of retinal nerve fiber layer defects: the Korea National Health and Nutrition Examination Survey 2008–2012. PLoS One. 2017; 12:e0186032.

15. Lee AJ, Wang JJ, Kifley A, Mitchell P. Open-angle glaucoma and cardiovascular mortality: the Blue Mountains Eye Study. Ophthalmology. 2006; 113:1069–1076.
16. Wu SY, Leske MC. Associations with intraocular pressure in the Barbados Eye Study. Arch Ophthalmol. 1997; 115:1572–1576.

17. Bulpitt CJ, Hodes C, Everitt MG. Intraocular pressure and systemic blood pressure in the elderly. Br J Ophthalmol. 1975; 59:717–720.

18. Leske MC, Podgor MJ. Intraocular pressure, cardiovascular risk variables, and visual field defects. Am J Epidemiol. 1983; 118:280–287.

19. Leske MC, Warheit-Roberts L, Wu SY. Open-angle glaucoma and ocular hypertension: the Long Island Glaucoma Case-control Study. Ophthalmic Epidemiol. 1996; 3:85–96.

20. Dielemans I, Vingerling JR, Algra D, et al. Primary open-angle glaucoma, intraocular pressure, and systemic blood pressure in the general elderly population. The Rotterdam Study. Ophthalmology. 1995; 102:54–60.
21. Bengtsson B. Some factors affecting the distribution of intraocular pressures in a population. Acta Ophthalmol (Copenh). 1972; 50:33–46.

22. Bonomi L, Marchini G, Marraffa M, et al. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology. 2000; 107:1287–1293.

23. Hennis A, Wu SY, Nemesure B, et al. Hypertension, diabetes, and longitudinal changes in intraocular pressure. Ophthalmology. 2003; 110:908–914.

24. Xu L, Wang H, Wang Y, Jonas JB. Intraocular pressure correlated with arterial blood pressure: the Beijing Eye Study. Am J Ophthalmol. 2007; 144:461–462.

26. Mitchell P, Leung H, Wang JJ, et al. Retinal vessel diameter and open-angle glaucoma: the Blue Mountains Eye Study. Ophthalmology. 2005; 112:245–250.
27. Hayreh SS. Posterior ciliary artery circulation in health and disease: the Weisenfeld lecture. Invest Ophthalmol Vis Sci. 2004; 45:749–757.

28. Hayreh SS, Zimmerman B. Visual field abnormalities in nonarteritic anterior ischemic optic neuropathy: their pattern and prevalence at initial examination. Arch Ophthalmol. 2005; 123:1554–1562.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download