Abstract
Purpose
Methods
Results
Figures and Tables
 | Fig. 2Secondary endpoints. (A) Changes in corneal fluorescein staining score from baseline (National Eye Institute [NEI] scale from 0 to 15). *p < 0.05. (B) Changes in conjunctival lissamine green staining score from baseline (NEI scale from 0 to 18). *p < 0.05. (C) Change in tear break-up time (TBUT) from baseline. *p < 0.05. (D) Changes in Schirmer test score from baseline. *p < 0.05. (E) Changes in Ocular Surface Disease Index (OSDI) score from baseline (OSDI score from 0 to 100). *p < 0.05. |
Table 1
Initial characteristics of each group with dry eye before treatment
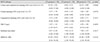
Values are presented as mean ± standard error (range).
CN group = Cyporin N 0.05% (cyclosporin nanoemulsion 0.5 mg/mL); CE group = Restasis 0.05% (cyclosporin emulsion 0.5 mg/mL); DQ group = Diquas 3% (Diquafosol sodium 30 mg/mL); NEI = National Eye Institute; TBUT = Tear break-up time; OSDI = Ocular Surface Disease Index.
*Comparison among three groups, Kruskal-Wallis test.
Table 2
Primary endpoint: changes in corneal and conjunctival staining score from baseline (NEI scale from 0 to 33)

Values are presented as mean ± standard error; Inferiority margin: −2.88.
NEI = National Eye Institute; CN group = Cyporin N 0.05% (cyclosporin nanoemulsion 0.5 mg/mL); CE group = Restasis 0.05% (cyclosporin emulsion 0.5 mg/mL); DQ group = Diquas 3% (Diquafosol sodium 30 mg/mL); CI = confidence interval.
*Comparison between CN and CE groups, Wilcoxon rank sum test; †Comparison between CN and DQ groups, Wilcoxon rank sum test; ‡Comparison between CE and DQ groups, Wilcoxon rank sum test; §Comparison among three groups, Kruskal-Wallis test.
Table 3
Comparisons of instillation number and adherence

Values are presented as mean ± standard error (range).
CN group = Cyporin N 0.05% (cyclosporin nanoemulsion 0.5 mg/mL); CE group = Restasis 0.05% (cyclosporin emulsion 0.5 mg/mL); DQ group = Diquas 3% (Diquafosol sodium 30 mg/mL).
*Comparison between CN and CE groups, Wilcoxon rank sum test; †Comparison between CN and DQ groups, Wilcoxon rank sum test; ‡Comparison between CE and DQ groups, Wilcoxon rank sum test; §Comparison among three groups, Kruskal-Wallis test.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download