This article has been
cited by other articles in ScienceCentral.
Abstract
A 64-year-old man presented with facial diplegia occurring 2 weeks after scrub typhus diagnosis. The serum scrub typhus antibody titer was elevated to 1:5120. Brain magnetic resonance imaging revealed contrast-enhancement of the signal for both facial nerves. He was administered prednisolone. After two weeks, the symptoms improved, and after one month, he completely recovered from facial diplegia. This is the first case in the literature in which the patient exhibited facial diplegia, a delayed complication, in scrub typhus. Facial diplegia should be considered a type of cranial nerve palsy that may occur as a delayed complication of scrub typhus.
Keywords: Facial palsy, Facial neuritis, Scrub typhus
Facial diplegia is very rare, and most cases of unilateral facial palsy are idiopathic. However, facial diplegia may often be accompanied by systemic diseases.
1 Scrub typhus refers to a systemic disease transmitted by
Orientia tsutsugamushi, and may cause neurological complications, including cranial nerve palsy, opsoclonus, Guillain-Barré syndrome (GBS), meningitis, and Parkinson's syndrome, but there is no report on whether it causes facial diplegia.
23
CASE
A 64-year-old man presented with facial diplegia. He had visited a primary healthcare center owing to fever, myalgia, and a skin rash that had appeared two weeks earlier. An eschar was found in the thigh area, and thus, he was diagnosed with scrub typhus. He was prescribed oral doxycycline (200 mg), and showed an improvement in symptoms over the 10 days of treatment. However, he suddenly developed facial diplegia 11 days after being diagnosed with scrub typhus. He did not have any other medical history; moreover, at the time of admission, he did not have a headache, and all his vital signs, including body temperature, were normal. Neurological tests showed that he was unable to completely close both his eyes. He exhibited reduced nasolabial folds. Facial sensation was normal, and there were no other neurological abnormalities. Laboratory tests yielded largely normal findings; however, the serum scrub typhus antibody titer was elevated to 1:5120. An examination of the cerebrospinal fluid (CSF) revealed pleocytosis and increased protein levels, with a pressure of 15 mmHg; white blood cell count of 20 cells/mm
3 (normal: 0–5/mm
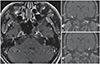
3), protein level of 94.5 mg/dl (normal: 15–50 mg/dl), and glucose level of 52 mg/dl (serum glucose: 121 mg/dl). Acid fast, Gram stain and culture, adenosine deaminase, herpes simplex and varicella-zoster virus, and bacteria and fungus tests for the CSF yielded normal results. However, the scrub typhus antibody titer was not examined in the CSF. Further, T1-weighted brain magnetic resonance imaging showed contrast enhancement of the signal for the facial nerves on both sides (
Fig. 1). Blink reflex showed poor wave formation for stimulation of both nerves. Stimulation of the left eye socket caused a delay in R1 and bilateral R2 response, and stimulation of the right eye socket caused a delay in R1 response but a normal bilateral R2 response (
Fig. 2). We subsequently performed a nerve conduction examination to test for GBS; the motor and sensory nerve conduction, F-wave, and H-reflex were normal. However, negative results were obtained for tests of anti-ganglioside antibodies, such as anti-GM1 and anti-GQ1b antibodies. He was administered oral prednisolone (60 mg). After 2 weeks of steroid therapy, the symptoms gradually improved, and after one month, he showed complete recovery from facial diplegia.
DISCUSSION
Herein, we report facial diplegia as a delayed complication of scrub typhus. Facial diplegia is a very rare illness and may have various etiologies.
1 Among infectious causes, Lyme disease is the most common; however, cases involving varicella-zoster, Epstein-Barr, polio, influenza, mumps, and coxsackie virus infections have also been reported.
4 An acute inflammatory demyelinating polyneuropathy-related autoimmune condition, GBS predominantly affects the peripheral nervous system.
5 It is characterized by flaccid paralysis of the extremities, with various degrees of cranial nerve involvement and isolated facial diplegia being observed in a few cases.
6 Other causes of facial diplegia include congenital and traumatic causes associated with temporal bone fracture.
7 The pathogenesis of facial diplegia includes direct pressure on the facial nerves and acute inflammation and edema in the these nerves, as well as their direct infiltration by toxins generated from a systemic disease or due to a secondary immune response caused by an infection.
4 Facial diplegia caused by scrub typhus is rare, and in most cases, occurs concomitantly with GBS, which may present various other forms of nerve palsy besides facial palsy.
389 This could be explained by the possibility that, in scrub typhus,
Orientia tsutsugamushi infiltrates the vascular endothelium of capillaries or small arteries, causing intrusion of inflammatory cells, which leads to vasculitis; another possibility is that it is caused by demyelination of the nerves due to a secondary immune response against an infection.
456 In the present case, the onset of facial diplegia occurred after a certain amount of time had passed since the appearance of symptoms caused by scrub typhus as well as contrast-enhanced magnetic resonance imaging findings in the facial nerves on both sides. There were no other neurological abnormalities, including sensory symptoms, and both R1 responses were delayed on the blink reflex test. Therefore, we presumed that immune response-associated inflammation and edema had occurred in the facial nerves. However, since the CSF was not tested scrub typhus antibody and tests for Lyme disease or sarcoidosis were not performed, the diagnosis may be limited in the present case.
In conclusion, we describe a case of facial diplegia, a delayed complication of scrub typhus, with no other causes. Facial diplegia should be considered a type of cranial nerve palsy that may occur as a delayed complication of scrub typhus.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download