Abstract
Primary ciliary dyskinesia (PCD) is a genetically heterogeneous disease affecting motile cilia. A female neonate was hospitalized with respiratory distress 72 hours after birth and showed concurrent situs inversus. She was identified to have compound heterozygous mutations in DNAH5: c.5647C>T, p.Arg1883Ter (nonsense mutation) and c.10810dupA, p.Ile3604AsnfsTer2 (frameshift mutation). Sanger sequencing confirmed that they were inherited from her father and mother, respectively, and she was diagnosed with PCD. The c.10810dupA is a novel DNAH5 mutation that has never been reported. To the best of our knowledge, this is the first report describing DNAH5 mutations in a Korean patient with PCD.
Figures and Tables
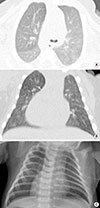 | Fig. 2(A, B) High resolution computed tomography scan shows diffuse low attenuation in right upper lobe without pneumonic infiltration. (C) Chest X-ray taken the same day. |
 | Fig. 3Genetic analysis revealed a heterozygous mutation in DNAH5: NM 001369.2:c.5647C>T, p.Arg1883Ter and NM 001369.2:c.10810dupA, p.Ile3604AsnfsTer2. Sanger sequencing confirmed that the patient's unaffected father carried NM 001369.2:c.5647C>T variant (nonsense mutation) (A) and the patient's unaffected mother carried NM 001369.2:c.10810dupA variant (frameshift mutation) that is a novel mutation in DNAH5 (B). |
References
1. Lucas JS, Burgess A, Mitchison HM, Moya E, Williamson M, Hogg C, et al. Diagnosis and management of primary ciliary dyskinesia. Arch Dis Child. 2014; 99:850–856.

2. Horani A, Ferkol TW. Advances in the genetics of primary ciliary dyskinesia: clinical implications. Chest. 2018; 154:645–652.

3. Davis SD, Ferkol TW, Rosenfeld M, Lee HS, Dell SD, Sagel SD, et al. Clinical features of childhood primary ciliary dyskinesia by genotype and ultrastructural phenotype. Am J Respir Crit Care Med. 2015; 191:316–324.

4. Takeuchi K, Kitano M, Ishinaga H, Kobayashi M, Ogawa S, Nakatani K, et al. Recent advances in primary ciliary dyskinesia. Auris Nasus Larynx. 2016; 43:229–236.

5. Horani A, Ferkol TW, Dutcher SK, Brody SL. Genetics and biology of primary ciliary dyskinesia. Paediatr Respir Rev. 2016; 18:18–24.

6. Raidt J, Wallmeier J, Hjeij R, Onnebrink JG, Pennekamp P, Loges NT, et al. Ciliary beat pattern and frequency in genetic variants of primary ciliary dyskinesia. Eur Respir J. 2014; 44:1579–1588.

7. Failly M, Bartoloni L, Letourneau A, Munoz A, Falconnet E, Rossier C, et al. Mutations in DNAH5 account for only 15% of a non-preselected cohort of patients with primary ciliary dyskinesia. J Med Genet. 2009; 46:281–286.

8. Zhang J, Guan L, Wen W, Lu Y, Zhu Q, Yuan H, et al. A novel mutation of DNAH5 in chronic rhinosinusitis and primary ciliary dyskinesia in a Chinese family. Eur Arch Otorhinolaryngol. 2014; 271:1589–1594.

9. Kano G, Tsujii H, Takeuchi K, Nakatani K, Ikejiri M, Ogawa S, et al. Whole-exome sequencing identification of novel DNAH5 mutations in a young patient with primary ciliary dyskinesia. Mol Med Rep. 2016; 14:5077–5083.

10. Hosie PH, Fitzgerald DA, Jaffe A, Birman CS, Rutland J, Morgan LC. Presentation of primary ciliary dyskinesia in children: 30 years' experience. J Paediatr Child Health. 2015; 51:722–726.

11. Mullowney T, Manson D, Kim R, Stephens D, Shah V, Dell S. Primary ciliary dyskinesia and neonatal respiratory distress. Pediatrics. 2014; 134:1160–1166.

12. Kennedy MP, Omran H, Leigh MW, Dell S, Morgan L, Molina PL, et al. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation. 2007; 115:2814–2821.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download